Alternative pharmacokinetics of S-1 components, 5-fluorouracil, dihydrofluorouracil and alpha-fluoro-beta-alanine after oral administration of S-1 following total gastrectomy
- PMID: 17683513
- PMCID: PMC11159371
- DOI: 10.1111/j.1349-7006.2007.00573.x
Alternative pharmacokinetics of S-1 components, 5-fluorouracil, dihydrofluorouracil and alpha-fluoro-beta-alanine after oral administration of S-1 following total gastrectomy
Abstract
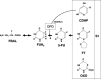
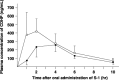
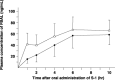
We studied whether total gastrectomy for gastric cancer would affect the pharmacokinetics of 5-fluorouracil (5-FU) and its degradation products, such as dihydrouracil (FUH(2)) and alpha-fluoro-beta-alanine (FBAL), after oral administration of the fluorouracil derivative S-1, composed of tegafur, 5-chloro-2,4-dihydroxypyridine (CDHP; a dihydropyrimidine dehydrogenase inhibitor) and potassium oxonate. Blood and urine samples were obtained, both preoperatively and at least 2 weeks postoperatively, from six patients with advanced gastric cancers who were undergoing total gastrectomy. Plasma levels of tegafur, 5-FU, CDHP, potassium oxonate, FUH(2) and FBAL were measured prior to and at 1, 2, 4, 6 and 10 h after oral administration of 40 mg/m(2) S-1. The total amounts of 5-FU, FUH(2) and FBAL excreted into urine during the 24-h period after S-1 administration were also measured. Total gastrectomy significantly increased the maximum concentration and the area under the curve until 10 h after administration (AUC(1-10h)) of plasma 5-FU. The plasma AUC(1-10h) of CDHP was significantly higher than the preoperative value. In terms of clinical efficacy, the higher AUC(1-10h) of 5-FU after total gastrectomy may be beneficial to S-1 administered as adjuvant chemotherapy, and might be caused by the higher postoperative AUC(1-10h) of CDHP relative to preoperative values. However, the dose of S-1 for patients who have undergone total gastrectomy might be diminished to avoid severe adverse events and to continue the treatment for a long period.
Figures




References
-
- Nagashima F, Ohtsu A, Yoshida S, Ito K. Japanese nationwide post‐marketing survey of S‐1 in patients with advanced gastric cancer. Gastric Cancer 2005; 8: 6–11. - PubMed
-
- Shirasaka T, Shimamoto Y, Fukushima M. Inhibition by oxonic acid of gastrointestinal toxicity of 5‐fluorouracil without loss of its antitumor activity in rats. Cancer Res 1993; 53: 4004–9. - PubMed
-
- Okeda R, Shibutani M, Matsuo T, Kuroiwa T, Shimokawa R, Tajima T. Experimental neurotoxicity of 5‐fluorouracil and its derivatives is due to poisoning by the monofluorinated organic metabolites, monofluoroacetic acid and α‐fluoro‐β‐alanine. Acta Neuropathol 1990; 81: 66–73. - PubMed
-
- Muneoka K, Shirai Y, Yokoyama N, Wakai T, Hatakeyama K. 5‐Fluorouracil cardiotoxicity induced by α‐fluoro‐β‐alanine. Int J Clin Oncol 2005; 10: 441–3. - PubMed
-
- Kinoshita T, Nashimoto A, Yamamura Y et al . Feasibility study of adjuvant chemotherapy with S‐1 (TS‐1; FT, gimeracil, oteracil potassium) for gastric cancer. Gastric Cancer 2004; 7: 104–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

