Pilot study of MDR1 gene transfer into hematopoietic stem cells and chemoprotection in metastatic breast cancer patients
- PMID: 17683514
- PMCID: PMC11158217
- DOI: 10.1111/j.1349-7006.2007.00571.x
Pilot study of MDR1 gene transfer into hematopoietic stem cells and chemoprotection in metastatic breast cancer patients
Abstract
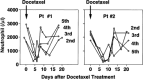
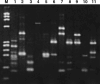
A major problem in high-dose chemotherapy with autologous hematopoietic stem cell transplantation is insufficient function of reconstituted bone marrow that limits the efficacy of post-transplantation chemotherapy. Because transduction of hematopoietic stem cells with the multidrug resistance 1 (MDR1) gene might circumvent this problem, we conducted a pilot study of MDR1 gene therapy against metastatic breast cancer. Peripheral blood stem cells were harvested, and one-third of the cells were transduced with MDR1 retrovirus. After the reconstitution of bone marrow function, the patients received high-dose chemotherapy with transplantation of both MDR1-transduced and unprocessed peripheral blood stem cells. The patients then received docetaxel chemotherapy. Two patients received transplantation of the MDR1-transduced cells in 2001. Peripheral blood MDR1-transduced leukocytes were 3-5% of the total cells after transplantation, but decreased gradually. During docetaxel chemotherapy, an increase in the rate of MDR1-transduced leukocytes (up to 10%) was observed. Comparison of docetaxel-induced granulocytopenia in the two patients suggested a bone marrow-protective effect of the MDR1-transduced cells. No serious side-effect was observed, and the patients were in complete remission for more than 3 years. The MDR1-transduced cells gradually decreased and disappeared almost entirely by the end of 2004. Results of linear amplification-mediated polymerase chain reaction of the MDR1-transduced leukocytes suggested no sign of abnormal amplification of the transduced cells. A third patient received transplantation of the MDR1-transduced cells in 2004. These results suggest the feasibility of our MDR1 gene therapy against metastatic breast cancer, and follow-up is ongoing.
Figures



References
-
- Inoue K, Ogawa M, Horikoshi N et al . Evaluation of prognostic factors for 233 patients with recurrent advanced breast cancer. Jpn J Clin Oncol 1991; 21: 334–9. - PubMed
-
- Philip T, Guglielmi C, Hagenbeek A et al . Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy‐sensitive non‐Hodgkin's lymphoma. N Engl J Med 1995; 333: 1540–5. - PubMed
-
- Attal M, Harousseau JL, Stoppa AM et al . A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med 1996; 335: 91–7. - PubMed
-
- Ito Y, Mukaiyama T, Ogawa M et al . Epirubicin‐containing high‐dose chemotherapy followed by autologous hematopoietic progenitor cell transfusion for patients with chemotherapy‐sensitive metastatic breast cancer: results of 5‐year follow‐up. Cancer Chemother Pharmacol 1999; 43: 8–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

