Adult autoimmune enteropathy: Mayo Clinic Rochester experience
- PMID: 17683994
- PMCID: PMC2128725
- DOI: 10.1016/j.cgh.2007.05.013
Adult autoimmune enteropathy: Mayo Clinic Rochester experience
Abstract
Background & aims: Autoimmune enteropathy is a rare cause of intractable diarrhea associated with circulating gut autoantibodies and a predisposition to autoimmunity. It is rarely observed in adults, with only 11 cases reported to date.
Methods: Fifteen adults with autoimmune enteropathy were identified at the Mayo Clinic, Rochester, from May 2001-June 2006. The demographic, clinical, and treatment data were abstracted from their records.
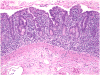
Results: The study population was 87% white, 47% female, with median age of 55 years (interquartile range, 42-67 years). All patients had protracted diarrhea, weight loss, and malnutrition. Celiac disease was excluded by lack of response to gluten-free diet or absence of the celiac disease susceptibility HLA genotypes. Fourteen patients were tested for gut epithelial cell antibodies, and 93% were positive for anti-enterocyte and/or anti-goblet cell antibodies. Predisposition to autoimmune diseases was noted in 80%, as indicated by a variety of circulating autoantibodies. Small intestinal histopathologic findings included subtotal villous atrophy and lymphoplasmacytic infiltration in the lamina propria with relatively few surface intraepithelial lymphocytes. T-cell receptor gene rearrangement studies were negative in all cases. Immunosuppressive therapy was required in 93% of cases. Clinical improvement was noted in 60% after 1-8 weeks of steroid therapy.
Conclusions: Autoimmune enteropathy is a heterogeneous disease and should be considered in the differential diagnosis of malabsorption and small bowel villous atrophy. The presence of gut epithelial cell antibodies can help confirm the diagnosis. No single agent is unequivocally effective in inducing remission, and immunosuppressive therapy is required in most cases.
Figures








References
-
- Walker-Smith JA, Unsworth DJ, Hutchins P, Phillips AD, Holborow EJ. Autoantibodies against gut epithelium in child with small-intestinal enteropathy. Lancet. 1982;1:566–7. - PubMed
-
- Unsworth DJ, Walker-Smith JA. Autoimmunity in diarrhoeal disease. J Pediatr Gastroenterol Nutr. 41985:375–80. - PubMed
-
- Ruemmele FM, Brousse N, Goulet O. Autoimmune enteropathy: molecular concepts. Curr Opin Gastroenterol. 2004;20:587–91. - PubMed
-
- Biagi F, Corazza GR. Defining gluten refractory enteropathy. Eur J Gastroenterol Hepatol. 2001;13:561–5. - PubMed
-
- Bousvaros A, Leichtner AM, Book L, Shigeoka A, Bilodeau J, Semeao E, Ruchelli E, Mulberg AE. Treatment of pediatric autoimmune enteropathy with tacrolimus (FK506) Gastroenterology. 1996;111:237–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

