Spatio-temporal analysis of eukaryotic cell motility by improved force cytometry
- PMID: 17684097
- PMCID: PMC1940228
- DOI: 10.1073/pnas.0705815104
Spatio-temporal analysis of eukaryotic cell motility by improved force cytometry
Abstract
Cell motility plays an essential role in many biological systems, but precise quantitative knowledge of the biophysical processes involved in cell migration is limited. Better measurements are needed to ultimately build models with predictive capabilities. We present an improved force cytometry method and apply it to the analysis of the dynamics of the chemotactic migration of the amoeboid form of Dictyostelium discoideum. Our explicit calculation of the force field takes into account the finite thickness of the elastic substrate and improves the accuracy and resolution compared with previous methods. This approach enables us to quantitatively study the differences in the mechanics of the migration of wild-type (WT) and mutant cell lines. The time evolution of the strain energy exerted by the migrating cells on their substrate is quasi-periodic and can be used as a simple indicator of the stages of the cell motility cycle. We have found that the mean velocity of migration v and the period of the strain energy T cycle are related through a hyperbolic law v = L/T, where L is a constant step length that remains unchanged in mutants with adhesion or contraction defects. Furthermore, when cells adhere to the substrate, they exert opposing pole forces that are orders of magnitude higher than required to overcome the resistance from their environment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

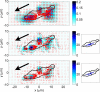


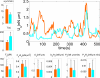


References
-
- Lauffenburger D, Horwitz A. Cell. 1996;84:359–369. - PubMed
-
- Pollard TD, Borisy GG. Cell. 2003;112:453–465. - PubMed
-
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Science. 2003;302:1704–1709. - PubMed
-
- Li S, Guan J, Chien S. Annu Rev Biomed Eng. 2005;7:105–150. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

