Evaluating "superiority", "equivalence" and "non-inferiority" in clinical trials
- PMID: 17684429
- PMCID: PMC6074290
- DOI: 10.5144/0256-4947.2007.284
Evaluating "superiority", "equivalence" and "non-inferiority" in clinical trials
Abstract
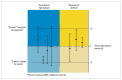
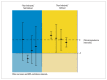
Clinical studies are usually performed with the aim of justifying that a new treatment approach is "superior" to the common standard approach (active control) with respect to benefits. In a general sense, this justification is carried out on the basis of the "null hypothesis significance test" with the P value based on this test used for justification. Today, new drugs differ so little from existing ones that factors such as cost and side effects affect the choice of therapy, when the bioavailability of treatment methods are found equivalent. Therefore, the aim of comparative clinical trials has extended beyond showing that a treatment is "superior" and now attempts to show that new treatments are "equal" and "non-inferior" to existing treatments. New approaches have become necessary since the classical null hypothesis approach is insufficient to justify the use of new agents, especially in cases of "equivalence" and "non-inferiority". This new approach to justification makes use of the "clinical equivalence interval", which determines the limits of the differences between specific endpoints that can be regarded as clinically "equal" to the value that was pre-specified based on studies of established therapies. It also makes use of the quantitative-based "confidence intervals" as the criteria for statistical justification. Many analyses can be done confidently when these tools are applied and the data are interpreted correctly.
Figures
Similar articles
-
[The importance of equivalence trials in showing the usefulness of treatments].Ned Tijdschr Geneeskd. 2003 Nov 1;147(44):2162-6. Ned Tijdschr Geneeskd. 2003. PMID: 14626832 Review. Dutch.
-
[Methodological and statistical aspects of equivalence and non inferiority trials].Rev Epidemiol Sante Publique. 2008 Aug;56(4):267-77. doi: 10.1016/j.respe.2008.05.027. Epub 2008 Aug 13. Rev Epidemiol Sante Publique. 2008. PMID: 18703296 Review. French.
-
Design and analysis of non-inferiority mortality trials in oncology.Stat Med. 2003 Jan 30;22(2):239-64. doi: 10.1002/sim.1400. Stat Med. 2003. PMID: 12520560
-
Methodological standards in non-inferiority AIDS trials: moving from adherence to compliance.BMC Med Res Methodol. 2006 Sep 20;6:46. doi: 10.1186/1471-2288-6-46. BMC Med Res Methodol. 2006. PMID: 16987409 Free PMC article.
-
[Methodology for superiority versus equivalence and non-inferior clinical studies. A practical review].Rev Med Inst Mex Seguro Soc. 2016 May-Jun;54(3):344-53. Rev Med Inst Mex Seguro Soc. 2016. PMID: 27100981 Review. Spanish.
Cited by
-
Statistical fundamentals on cancer research for clinicians: Working with your statisticians.Clin Transl Radiat Oncol. 2021 Jan 16;27:75-84. doi: 10.1016/j.ctro.2021.01.006. eCollection 2021 Mar. Clin Transl Radiat Oncol. 2021. PMID: 33532634 Free PMC article. Review.
-
Small incision lenticule extraction (SMILE) versus laser in-situ keratomileusis (LASIK): study protocol for a randomized, non-inferiority trial.Trials. 2012 May 31;13:75. doi: 10.1186/1745-6215-13-75. Trials. 2012. PMID: 22647480 Free PMC article. Clinical Trial.
References
-
- Gomberg-Maitland M, Frison L, Halperin JL. Active-control clinical trials to esteblish equivalence or noninferiority: Methodological and statistical concepts linked to quality. Am Hearth J. 2003;146:398–403. - PubMed
-
- Temple R, Ellenberg SS. Placebo-controlled trials and active-control trials in the evaluation of new treatments. Annals of International Medicine. 2000;133(6):455–63. - PubMed
-
- McAlister FA, Sackett DL. Active-control equivalence trials and antihypertensive agents. Am J Med. 2001;111:553–8. - PubMed
-
- Committee for Proprietary Medicinal Products (CPMP) Points to Consider on the Choice of Non-inferiority Magrin; London. 2004 February 26; CPMP/EWP/2158/99 draft.
-
- Committee for Proprietary Medicinal Products (CPMP) Points to Consider on Switching Between Superiority and Non-inferiority; London. 2000 July 27; CPMP/EWP/482/99.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical