Disparate effects of p24alpha and p24delta on secretory protein transport and processing
- PMID: 17684551
- PMCID: PMC1933603
- DOI: 10.1371/journal.pone.0000704
Disparate effects of p24alpha and p24delta on secretory protein transport and processing
Abstract
Background: The p24 family is thought to be somehow involved in endoplasmic reticulum (ER)-to-Golgi protein transport. A subset of the p24 proteins (p24alpha(3), -beta(1), -gamma(3) and -delta(2)) is upregulated when Xenopus laevis intermediate pituitary melanotrope cells are physiologically activated to produce vast amounts of their major secretory cargo, the prohormone proopiomelanocortin (POMC).
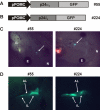
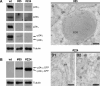
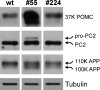
Methodology/principal findings: Here we find that transgene expression of p24alpha(3 )or p24delta(2) specifically in the Xenopus melanotrope cells in both cases causes an effective displacement of the endogenous p24 proteins, resulting in severely distorted p24 systems and disparate melanotrope cell phenotypes. Transgene expression of p24alpha(3) greatly reduces POMC transport and leads to accumulation of the prohormone in large, ER-localized electron-dense structures, whereas p24delta(2)-transgenesis does not influence the overall ultrastructure of the cells nor POMC transport and cleavage, but affects the Golgi-based processes of POMC glycomaturation and sulfation.
Conclusions/significance: Transgenic expression of two distinct p24 family members has disparate effects on secretory pathway functioning, illustrating the specificity and non-redundancy of our transgenic approach. We conclude that members of the p24 family furnish subcompartments of the secretory pathway with specific sets of machinery cargo to provide the proper microenvironments for efficient and correct secretory protein transport and processing.
Conflict of interest statement
Figures

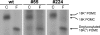

Similar articles
-
Functional diversity among p24 subfamily members.Biol Cell. 2009 Apr;101(4):207-19. doi: 10.1042/BC20080075. Biol Cell. 2009. PMID: 18699773
-
COP-binding sites in p24delta2 are necessary for proper secretory cargo biosynthesis.Int J Biochem Cell Biol. 2009 Jul;41(7):1619-27. doi: 10.1016/j.biocel.2009.02.010. Epub 2009 Feb 24. Int J Biochem Cell Biol. 2009. PMID: 19401156
-
p24 Proteins from the same subfamily are functionally nonredundant.Biochimie. 2011 Mar;93(3):528-32. doi: 10.1016/j.biochi.2010.11.007. Epub 2010 Nov 29. Biochimie. 2011. PMID: 21118709
-
Using transgenic animal models in neuroendocrine research: lessons from Xenopus laevis.Ann N Y Acad Sci. 2009 Apr;1163:296-307. doi: 10.1111/j.1749-6632.2008.03644.x. Ann N Y Acad Sci. 2009. PMID: 19456351 Review.
-
The p24 family and selective transport processes at the ER-Golgi interface.Biol Cell. 2009 Sep;101(9):495-509. doi: 10.1042/BC20080233. Biol Cell. 2009. PMID: 19566487 Review.
Cited by
-
Function of a p24 Heterodimer in Morphogenesis and Protein Transport in Penicillium oxalicum.Sci Rep. 2015 Jul 7;5:11875. doi: 10.1038/srep11875. Sci Rep. 2015. PMID: 26149342 Free PMC article.
-
V-ATPase-mediated granular acidification is regulated by the V-ATPase accessory subunit Ac45 in POMC-producing cells.Mol Biol Cell. 2010 Oct 1;21(19):3330-9. doi: 10.1091/mbc.E10-04-0274. Epub 2010 Aug 11. Mol Biol Cell. 2010. PMID: 20702583 Free PMC article.
-
Transmembrane emp24 domain proteins in development and disease.Genet Res (Camb). 2019 Dec 27;101:e14. doi: 10.1017/S0016672319000090. Genet Res (Camb). 2019. PMID: 31878985 Free PMC article. Review.
-
Incomplete posttranslational prohormone modifications in hyperactive neuroendocrine cells.BMC Cell Biol. 2009 May 7;10:35. doi: 10.1186/1471-2121-10-35. BMC Cell Biol. 2009. PMID: 19422674 Free PMC article.
-
Isoform-selective oligomer formation of Saccharomyces cerevisiae p24 family proteins.J Biol Chem. 2013 Dec 27;288(52):37057-70. doi: 10.1074/jbc.M113.518340. Epub 2013 Nov 11. J Biol Chem. 2013. PMID: 24217251 Free PMC article.
References
-
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975;189:347–358. - PubMed
-
- Carney GE, Bowen NJ. p24 proteins, intracellular trafficking, and behavior: Drosophila melanogaster provides insights and opportunities. Biol Cell. 2004;96:271–278. - PubMed
-
- Emery G, Gruenberg J, Rojo M. The p24 family of transmembrane proteins at the interface between endoplasmic reticulum and Golgi apparatus. Protoplasma. 1999;V207:24–30.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

