The murine gammaherpesvirus-68 gp150 acts as an immunogenic decoy to limit virion neutralization
- PMID: 17684552
- PMCID: PMC1931612
- DOI: 10.1371/journal.pone.0000705
The murine gammaherpesvirus-68 gp150 acts as an immunogenic decoy to limit virion neutralization
Abstract
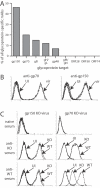
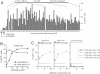
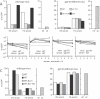
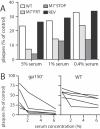
Herpesviruses maintain long-term infectivity without marked antigenic variation. They must therefore evade neutralization by other means. Immune sera block murine gammaherpesvirus-68 (MHV-68) infection of fibroblasts, but fail to block and even enhance its infection of IgG Fc receptor-bearing cells, suggesting that the antibody response to infection is actually poor at ablating virion infectivity completely. Here we analyzed this effect further by quantitating the glycoprotein-specific antibody response of MHV-68 carrier mice. Gp150 was much the commonest glycoprotein target and played a predominant role in driving Fc receptor-dependent infection: when gp150-specific antibodies were boosted, Fc receptor-dependent infection increased; and when gp150-specific antibodies were removed, Fc receptor-dependent infection was largely lost. Neither gp150-specific monoclonal antibodies nor gp150-specific polyclonal sera gave significant virion neutralization. Gp150 therefore acts as an immunogenic decoy, distorting the MHV-68-specific antibody response to promote Fc receptor-dependent infection and so compromise virion neutralization. This immune evasion mechanism may be common to many non-essential herpesvirus glycoproteins.
Conflict of interest statement
Figures


References
-
- Zinkernagel RM, Hengartner H. Protective ‘immunity’ by pre-existent neutralizing antibody titers and preactivated T cells but not by so-called ‘immunological memory’. Immunol Rev. 2006;211:310–319. - PubMed
-
- Sissons JG, Oldstone MB. Killing of virus-infected cells: the role of antiviral antibody and complement in limiting virus infection. J Infect Dis. 1980;142:442–448. - PubMed
-
- Xu J, Lyons PA, Carter MD, Booth TW, Davis-Poynter NJ, et al. Assessment of antigenicity and genetic variation of glycoprotein B of murine cytomegalovirus. J Gen Virol. 1996;77:49–59. - PubMed

