Systems analysis of N-glycan processing in mammalian cells
- PMID: 17684559
- PMCID: PMC1933599
- DOI: 10.1371/journal.pone.0000713
Systems analysis of N-glycan processing in mammalian cells
Abstract
N-glycosylation plays a key role in the quality of many therapeutic glycoprotein biologics. The biosynthesis reactions of these oligosaccharides are a type of network in which a relatively small number of enzymes give rise to a large number of N-glycans as the reaction intermediates and terminal products. Multiple glycans appear on the glycoprotein molecules and give rise to a heterogeneous product. Controlling the glycan distribution is critical to the quality control of the product. Understanding N-glycan biosynthesis and the etiology of microheterogeneity would provide physiological insights, and facilitate cellular engineering to enhance glycoprotein quality. We developed a mathematical model of glycan biosynthesis in the Golgi and analyzed the various reaction variables on the resulting glycan distribution. The Golgi model was modeled as four compartments in series. The mechanism of protein transport across the Golgi is still controversial. From the viewpoint of their holding time distribution characteristics, the two main hypothesized mechanisms, vesicular transport and Golgi maturation models, resemble four continuous mixing-tanks (4CSTR) and four plug-flow reactors (4PFR) in series, respectively. The two hypotheses were modeled accordingly and compared. The intrinsic reaction kinetics were first evaluated using a batch (or single PFR) reactor. A sufficient holding time is needed to produce terminally-processed glycans. Altering enzyme concentrations has a complex effect on the final glycan distribution, as the changes often affect many reaction steps in the network. Comparison of the glycan profiles predicted by the 4CSTR and 4PFR models points to the 4PFR system as more likely to be the true mechanism. To assess whether glycan heterogeneity can be eliminated in the biosynthesis of biotherapeutics the 4PFR model was further used to assess whether a homogeneous glycan profile can be created through metabolic engineering. We demonstrate by the spatial localization of enzymes to specific compartments all terminally processed N-glycans can be synthesized as homogeneous products with a sufficient holding time in the Golgi compartments. The model developed may serve as a guide to future engineering of glycoproteins.
Conflict of interest statement
Figures


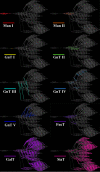

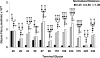

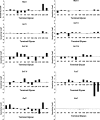


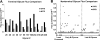

References
-
- Seth G, Hossler P, Yee JC, Hu WS. Engineering cells for cell culture bioprocessing–physiological fundamentals. Adv Biochem Eng Biotechnol. 2006;101:119–164. - PubMed
-
- Sinclair AM, Elliott S. Glycoengineering: the effect of glycosylation on the properties of therapeutic proteins. J Pharm Sci. 2005;94:1626–1635. - PubMed
-
- Carraway KL, Hull SR. O-glycosylation pathway for mucin-type glycoproteins. Bioessays. 1989;10:117–121. - PubMed
-
- Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. - PubMed
-
- Hebert DN, Garman SC, Molinari M. The glycan code of the endoplasmic reticulum: asparagine-linked carbohydrates as protein maturation and quality-control tags. Trends Cell Biol. 2005;15:364–370. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

