Evidence for differential changes of junctional complex proteins in murine neurocysticercosis dependent upon CNS vasculature
- PMID: 17686468
- PMCID: PMC2754301
- DOI: 10.1016/j.brainres.2007.07.010
Evidence for differential changes of junctional complex proteins in murine neurocysticercosis dependent upon CNS vasculature
Abstract
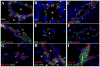
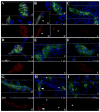
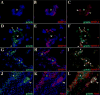
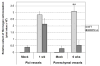
The delicate balance required to maintain homeostasis of the central nervous system (CNS) is controlled by the blood-brain barrier (BBB). Upon injury, the BBB is disrupted compromising the CNS. BBB disruption has been represented as a uniform event. However, our group has shown in a murine model of neurocysticercosis (NCC) that BBB disruption varies depending upon the anatomical site/vascular bed analyzed. In this study further understanding of the mechanisms of BBB disruption was explored in blood vessels located in leptomeninges (pial vessels) and brain parenchyma (parenchymal vessels) by examining the expression of junctional complex proteins in murine brain infected with Mesocestoides corti. Both pial and parenchymal vessels from mock infected animals showed significant colocalization of junctional proteins and displayed an organized architecture. Upon infection, the patterned organization was disrupted and in some cases, particular tight junction and adherens junction proteins were undetectable or appeared to be undergoing proteolysis. The extent and timing of these changes differed between both types of vessels (pial vessel disruption within days versus weeks for parenchymal vessels). To approach potential mechanisms, the expression and activity of matrix metalloproteinase-9 (MMP-9) were evaluated by in situ zymography. The results indicated an increase in MMP-9 activity at sites of BBB disruption exhibiting leukocyte infiltration. Moreover, the timing of MMP activity in pial and parenchymal vessels correlated with the timing of permeability disruption. Thus, breakdown of the BBB is a mutable process despite the similar structure of the junctional complex between pial and parenchymal vessels and involvement of MMP activity.
Figures


References
-
- Allt G, Lawrenson JG. Is the pial microvessel a good model for blood-brain barrier studies? Brain Res Brain Res Rev. 1997;24:67–76. - PubMed
-
- Alvarez JI, Teale JM. Breakdown of the blood brain barrier and blood-cerebrospinal fluid barrier is associated with differential leukocyte migration in distinct compartments of the CNS during the course of murine NCC. J Neuroimmunol. 2006;173:45–55. - PubMed
-
- Andras IE, Pu H, Deli MA, Nath A, Hennig B, Toborek M. HIV-1 Tat protein alters tight junction protein expression and distribution in cultured brain endothelial cells. J Neurosci Res. 2003;74:255–65. - PubMed
-
- Aurrand-Lions M, Johnson-Leger C, Wong C, Du Pasquier L, Imhof BA. Heterogeneity of endothelial junctions is reflected by differential expression and specific subcellular localization of the three JAM family members. Blood. 2001;98:3699–707. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

