LXXLL motifs and AF-2 domain mediate SHP (NR0B2) homodimerization and DAX1 (NR0B1)-DAX1A heterodimerization
- PMID: 17686645
- PMCID: PMC2065763
- DOI: 10.1016/j.ymgme.2007.06.009
LXXLL motifs and AF-2 domain mediate SHP (NR0B2) homodimerization and DAX1 (NR0B1)-DAX1A heterodimerization
Abstract
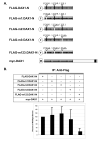
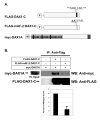
Small heterodimer partner (SHP; NR0B2) is an unusual orphan member of the nuclear receptor superfamily that functions as a corepressor of other nuclear receptors through heterodimeric interactions. Mutations in SHP are associated with mild obesity and insulin resistance. The protein domain structure of SHP is similar to Dosage-sensitive sex reversal adrenal hypoplasia congenita (AHC) critical region on the X chromosome, gene 1 (DAX1; NR0B1). Mutations in DAX1 cause AHC with associated hypogonadotropic hypogonadism. DAX1A is an alternatively spliced isoform of DAX1 that lacks the last 80 amino acids of the DAX1 C-terminal repressor domain and is replaced by a novel 10-amino acid motif. We have previously shown homodimerization of SHP and DAX1 individually, heterodimerization of DAX1 with SHP, and heterodimerization of DAX1 with DAX1A. In these studies, we investigated the domains and residues of SHP involved in SHP homodimerization and DAX1-SHP heterodimerization and also further characterized DAX1-DAX1 homodimerization and DAX1-DAX1A heterodimerization. We showed involvement of the SHP LXXLL motifs and AF-2 domain in SHP homodimerization and DAX1-SHP heterodimerization. We demonstrated redundancy of the LXXLL motifs in DAX1 homodimerization. While DAX1A subcellular localization is mostly cytoplasmic, DAX1-DAX1A heterodimers existed in the nucleus, suggesting differential functions for DAX1A in each compartment. We showed that the AF-2 domain of DAX1 is involved in DAX1-DAX1A heterodimerization. These results indicate that NR0B family members use similar mechanisms for homodimerization as well as heterodimerization. These resemble coactivator-receptor interactions that may have potential functional consequences for molecular mechanisms of the NR0B family.
Figures




Similar articles
-
Dosage-sensitive sex reversal adrenal hypoplasia congenita critical region on the X chromosome, gene 1 (DAX1) (NR0B1) and small heterodimer partner (SHP) (NR0B2) form homodimers individually, as well as DAX1-SHP heterodimers.Mol Endocrinol. 2006 Oct;20(10):2326-42. doi: 10.1210/me.2005-0383. Epub 2006 May 18. Mol Endocrinol. 2006. PMID: 16709599
-
DAX1: Increasing complexity in the roles of this novel nuclear receptor.Mol Cell Endocrinol. 2007 Feb;265-266:179-82. doi: 10.1016/j.mce.2006.12.017. Epub 2007 Jan 8. Mol Cell Endocrinol. 2007. PMID: 17210221 Free PMC article. Review.
-
NR0B1A: an alternatively spliced form of NR0B1.Mol Genet Metab. 2004 Dec;83(4):330-6. doi: 10.1016/j.ymgme.2004.10.002. Mol Genet Metab. 2004. PMID: 15589120
-
Genome-wide analysis of LXXLL-mediated DAX1/SHP-nuclear receptor interaction network and rational design of stapled LXXLL-based peptides to target the specific network profile.Int J Biol Macromol. 2019 May 15;129:13-22. doi: 10.1016/j.ijbiomac.2019.02.014. Epub 2019 Feb 4. Int J Biol Macromol. 2019. PMID: 30731167
-
Phenotypic spectrum of mutations in DAX-1 and SF-1.Mol Cell Endocrinol. 2001 Dec 20;185(1-2):17-25. doi: 10.1016/s0303-7207(01)00619-0. Mol Cell Endocrinol. 2001. PMID: 11738790 Review.
Cited by
-
Identification of common and cell type specific LXXLL motif EcR cofactors using a bioinformatics refined candidate RNAi screen in Drosophila melanogaster cell lines.BMC Dev Biol. 2011 Nov 3;11:66. doi: 10.1186/1471-213X-11-66. BMC Dev Biol. 2011. PMID: 22050674 Free PMC article.
-
A pleiotropic role for the orphan nuclear receptor small heterodimer partner in lipid homeostasis and metabolic pathways.J Lipids. 2012;2012:304292. doi: 10.1155/2012/304292. Epub 2012 Apr 22. J Lipids. 2012. PMID: 22577560 Free PMC article.
-
Inhibition of GLI Transcriptional Activity and Prostate Cancer Cell Growth and Proliferation by DAX1.Curr Issues Mol Biol. 2023 Jun 27;45(7):5347-5361. doi: 10.3390/cimb45070339. Curr Issues Mol Biol. 2023. PMID: 37504255 Free PMC article.
-
Small heterodimer partner 1 directly interacts with NS5A viral protein and has a key role in HCV related liver cell transformation.Oncotarget. 2016 Dec 20;7(51):84575-84586. doi: 10.18632/oncotarget.12144. Oncotarget. 2016. PMID: 27661118 Free PMC article.
-
Inhibition of β-catenin dependent WNT signalling upregulates the transcriptional repressor NR0B1 and downregulates markers of an A9 phenotype in human embryonic stem cell-derived dopaminergic neurons: Implications for Parkinson's disease.PLoS One. 2021 Dec 23;16(12):e0261730. doi: 10.1371/journal.pone.0261730. eCollection 2021. PLoS One. 2021. PMID: 34941945 Free PMC article.
References
-
- Seol W, Choi HS, Moore DD. An orphan nuclear hormone receptor that lacks a DNA binding domain and heterodimerizes with other receptors. Science. 1996;272:1336–1339. - PubMed
-
- Burris TP. The nuclear receptor superfamily. In: Burris TP, McCabe ERB, editors. Nuclear Receptors and Genetic Disease. Academic Press; San Diego: 2001. pp. 1–57.
-
- Giguere V. Orphan nuclear receptors: from gene to function. Endocr Rev. 1999;20:689–725. - PubMed
-
- McCabe ERB. Adrenal hypoplasias and aplasias. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Vogelstein B, editors. The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; New York: 2001. pp. 4263–4274.
-
- Ho J, Zhang YH, Huang BL, McCabe ER. NR0B1A: an alternatively spliced form of NR0B1. Mol Genet Metab. 2004;83:330–336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

