Tissue-specific splicing regulator Fox-1 induces exon skipping by interfering E complex formation on the downstream intron of human F1gamma gene
- PMID: 17686786
- PMCID: PMC2018636
- DOI: 10.1093/nar/gkm569
Tissue-specific splicing regulator Fox-1 induces exon skipping by interfering E complex formation on the downstream intron of human F1gamma gene
Abstract
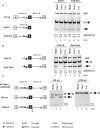
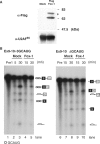
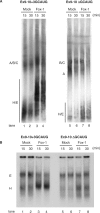
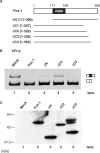
Fox-1 is a regulator of tissue-specific splicing, via binding to the element (U)GCAUG in mRNA precursors, in muscles and neuronal cells. Fox-1 can regulate splicing positively or negatively, most likely depending on where it binds relative to the regulated exon. In cases where the (U)GCAUG element lies in an intron upstream of the alternative exon, Fox-1 protein functions as a splicing repressor to induce exon skipping. Here we report the mechanism of exon skipping regulated by Fox-1, using the hF1gamma gene as a model system. We found that Fox-1 induces exon 9 skipping by repressing splicing of the downstream intron 9 via binding to the GCAUG repressor elements located in the upstream intron 8. In vitro splicing analyses showed that Fox-1 prevents formation of the pre-spliceosomal early (E) complex on intron 9. In addition, we located a region of the Fox-1 protein that is required for inducing exon skipping. Taken together, our data show a novel mechanism of how RNA-binding proteins regulate alternative splicing.
Figures

Similar articles
-
U1-independent pre-mRNA splicing contributes to the regulation of alternative splicing.Nucleic Acids Res. 2009 Apr;37(6):1907-14. doi: 10.1093/nar/gkp050. Epub 2009 Feb 3. Nucleic Acids Res. 2009. PMID: 19190090 Free PMC article.
-
A neuron-specific splicing switch mediated by an array of pre-mRNA repressor sites: evidence of a regulatory role for the polypyrimidine tract binding protein and a brain-specific PTB counterpart.RNA. 1997 Sep;3(9):996-1015. RNA. 1997. PMID: 9292499 Free PMC article.
-
Acidic stimulation induces a negative regulatory factor that affects alternative exon selection in vitro in human ATP synthase gamma-subunit pre-mRNA.Biochem Biophys Res Commun. 1998 Oct 20;251(2):603-8. doi: 10.1006/bbrc.1998.9525. Biochem Biophys Res Commun. 1998. PMID: 9792820
-
Fox-1 family of RNA-binding proteins.Cell Mol Life Sci. 2009 Dec;66(24):3895-907. doi: 10.1007/s00018-009-0120-5. Cell Mol Life Sci. 2009. PMID: 19688295 Free PMC article. Review.
-
Exon and intron definition in pre-mRNA splicing.Wiley Interdiscip Rev RNA. 2013 Jan-Feb;4(1):49-60. doi: 10.1002/wrna.1140. Epub 2012 Oct 8. Wiley Interdiscip Rev RNA. 2013. PMID: 23044818 Review.
Cited by
-
A pathway involving HDAC5, cFLIP and caspases regulates expression of the splicing regulator polypyrimidine tract binding protein in the heart.J Cell Sci. 2013 Apr 1;126(Pt 7):1682-91. doi: 10.1242/jcs.121384. Epub 2013 Feb 19. J Cell Sci. 2013. PMID: 23424201 Free PMC article.
-
Rbfox2-coordinated alternative splicing of Mef2d and Rock2 controls myoblast fusion during myogenesis.Mol Cell. 2014 Aug 21;55(4):592-603. doi: 10.1016/j.molcel.2014.06.035. Epub 2014 Jul 31. Mol Cell. 2014. PMID: 25087874 Free PMC article.
-
Rbfox1 is required for myofibril development and maintaining fiber type-specific isoform expression in Drosophila muscles.Life Sci Alliance. 2022 Jan 7;5(4):e202101342. doi: 10.26508/lsa.202101342. Print 2022 Apr. Life Sci Alliance. 2022. PMID: 34996845 Free PMC article.
-
Cytosolic localization of Fox proteins in motor neurons of G93A SOD1 mice.Histochem Cell Biol. 2016 May;145(5):545-59. doi: 10.1007/s00418-015-1393-4. Epub 2016 Jan 2. Histochem Cell Biol. 2016. PMID: 26724814
-
Complex Phenotype of a Boy With De Novo 16p13.3-13.2 Interstitial Deletion.Child Neurol Open. 2016 Dec 16;3:2329048X16676153. doi: 10.1177/2329048X16676153. eCollection 2016 Jan-Dec. Child Neurol Open. 2016. PMID: 28503620 Free PMC article.
References
-
- Graveley BR. Alternative splicing: increasing diversity in the proteomic world. Trends Genet. 2001;17:100–107. - PubMed
-
- Maniatis T, Tasic B. Alternative pre-mRNA splicing and proteome expansion in metazoans. Nature. 2002;418:236–243. - PubMed
-
- Black DL. Mechanisms of alternative pre-messenger RNA splicing. Annu. Rev. Biochem. 2003;72:291–336. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

