Intracellular trafficking and maturation of herpes simplex virus type 1 gB and virus egress require functional biogenesis of multivesicular bodies
- PMID: 17686835
- PMCID: PMC2045546
- DOI: 10.1128/JVI.01364-07
Intracellular trafficking and maturation of herpes simplex virus type 1 gB and virus egress require functional biogenesis of multivesicular bodies
Abstract
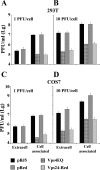
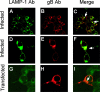
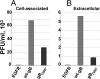
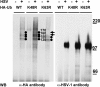
The biogenesis of multivesicular bodies (MVBs) is topologically equivalent to virion budding. Hence, a number of viruses exploit the MVB pathway to build their envelope and exit from the cell. By expression of dominant negative forms of Vps4 and Vps24, two components of the MVB pathway, we observed an impairment in infectious herpes simplex virus (HSV) assembly/egress, in agreement with a recent report showing the involvement in HSV envelopment of Vps4, the MVB-specific ATPase (C. M. Crump, C. Yates, and T. Minson, J. Virol. 81:7380-7387). Furthermore, HSV infection resulted in morphological changes to MVBs. Glycoprotein B (gB), one of the most highly conserved glycoproteins across the Herpesviridae family, was sorted to MVB membranes. In cells expressing the dominant negative form of Vps4, the site of intracellular gB accumulation was altered; part of gB accumulated as an endoglycosidase H-sensitive immature form at a calreticulin-positive compartment, indicating that gB traffic was dependent on a functional MVB pathway. gB was ubiquitinated in both infected and transfected cells. Ubiquitination was in part dependent on ubiquitin lysine 63, a signal for cargo sorting to MVBs. Partial deletion of the gB cytoplasmic tail resulted in a dramatic reduction of ubiquitination, as well as of progeny virus assembly and release to the extracellular compartment. Thus, HSV envelopment/egress and gB intracellular trafficking are dependent on functional MVB biogenesis. Our data support the view that the sorting of gB to MVB membranes may represent a critical step in HSV envelopment and egress and that modified MVB membranes constitute a platform for HSV cytoplasmic envelopment or that MVB components are recruited to the site(s) of envelopment.
Figures




References
-
- Avitabile, E., G. Lombardi, T. Gianni, M. Capri, and G. Campadelli-Fiume. 2004. Coexpression of UL20p and gK inhibits cell-cell fusion mediated by herpes simplex virus glycoproteins gD, gH-gL, and wild-type gB or an endocytosis-defective gB mutant and downmodulates their cell surface expression. J. Virol. 78:8015-8080. - PMC - PubMed
-
- Babst, M. 2005. A protein's final ESCRT. Traffic 6:2-9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

