Torque teno virus (SANBAN isolate) ORF2 protein suppresses NF-kappaB pathways via interaction with IkappaB kinases
- PMID: 17686849
- PMCID: PMC2168763
- DOI: 10.1128/JVI.01101-07
Torque teno virus (SANBAN isolate) ORF2 protein suppresses NF-kappaB pathways via interaction with IkappaB kinases
Erratum in
- J Virol. 2008 Jan;82(1):593
Abstract
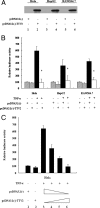
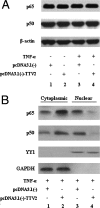
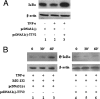
Since the first discovery of Torque teno virus (TTV) in 1997, many researchers focused on its epidemiology and transcriptional regulation, but the function of TTV-encoded proteins remained unknown. The function of the TTV open reading frame (ORF) in the nuclear factor kappaB (NF-kappaB) pathway has not yet been established. In this study, we found for the first time that the TTV ORF2 protein could suppress NF-kappaB activity in a dose-dependent manner in the canonical NF-kappaB pathway. By Western blot analysis, we proved that the TTV ORF2 protein did not alter the level of NF-kappaB expression but prevented the p50 and p65 subunits from entering the nucleus due to the inhibition of IkappaBalpha protein degradation. Further immunoprecipitation assays showed that the TTV ORF2 protein could physically interact with IKKbeta as well as IKKalpha, but not IKKgamma. Luciferase assays and Western blot experiments showed that the TTV ORF2 protein could also suppress NF-kappaB activity in the noncanonical NF-kappaB pathway and block the activation and translocation of p52. Finally, we found that the TTV ORF2 protein inhibited the transcription of NF-kappaB-mediated downstream genes (interleukin 6 [IL-6], IL-8, and COX-2) through down-regulation of NF-kappaB. Together, these data indicate that the TTV ORF2 protein suppresses the canonical and noncanonical NF-kappaB pathways, suggesting that the TTV ORF2 protein may be involved in regulating the innate and adaptive immunity of organisms, contributing to TTV pathogenesis, and even be related to some diseases.
Figures

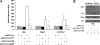

References
-
- Aggarwal, B. B. 2004. Nuclear factor-kappaB: the enemy within. Cancer Cell 6:203-208. - PubMed
-
- Aggarwal, B. B., S. Shishodia, S. K. Sandur, M. K. Pandey, and G. Sethi. 2006. Inflammation and cancer: how hot is the link? Biochem. Pharmacol. 72:1605-1621. - PubMed
-
- Angelo, L. S., M. Talpaz, and R. Kurzrock. 2002. Autocrine interleukin-6 production in renal cell carcinoma: evidence for the involvement of p53. Cancer Res. 62:932-940. - PubMed
-
- Baeuerle, P. A., and D. Baltimore. 1988. I kappa B: a specific inhibitor of the NF-kappa B transcription factor. Science 242:540-546. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

