Characterization of human immunodeficiency virus type 1 replication in immature and mature dendritic cells reveals dissociable cis- and trans-infection
- PMID: 17686876
- PMCID: PMC2045571
- DOI: 10.1128/JVI.01081-07
Characterization of human immunodeficiency virus type 1 replication in immature and mature dendritic cells reveals dissociable cis- and trans-infection
Abstract
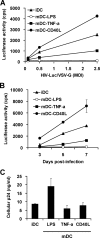
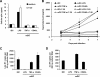
Dendritic cells (DCs) transmit human immunodeficiency virus type 1 (HIV-1) to CD4(+) T cells through the trans- and cis-infection pathways; however, little is known about the relative efficiencies of these pathways and whether they are interdependent. Here we compare cis- and trans-infections of HIV-1 mediated by immature DCs (iDCs) and mature DCs (mDCs), using replication-competent and single-cycle HIV-1. Monocyte-derived iDCs were differentiated into various types of mDCs by lipopolysaccharide (LPS), tumor necrosis factor alpha (TNF-alpha), and CD40 ligand (CD40L). iDCs and CD40L-induced mDCs were susceptible to HIV-1 infection and mediated efficient viral transmission to CD4(+) T cells. Although HIV-1 cis-infection was partially restricted in TNF-alpha-induced mDCs and profoundly blocked in LPS-induced mDCs, these cells efficiently promoted HIV-1 trans-infection of CD4(+) T cells. The postentry restriction of HIV-1 infection in LPS-induced mDCs was identified at the levels of reverse transcription and postintegration, using real-time PCR quantification of viral DNA and integration. Furthermore, nucleofection of DCs with HIV-1 proviral DNA confirmed that impaired gene expression of LPS-induced mDCs was responsible for the postentry restriction of HIV-1 infection. Our results suggest that various DC subsets in vivo may differentially contribute to HIV-1 dissemination via dissociable cis- and trans-infections.
Figures





Similar articles
-
Different Expression of Interferon-Stimulated Genes in Response to HIV-1 Infection in Dendritic Cells Based on Their Maturation State.J Virol. 2017 Mar 29;91(8):e01379-16. doi: 10.1128/JVI.01379-16. Print 2017 Apr 15. J Virol. 2017. PMID: 28148784 Free PMC article.
-
Functionally distinct transmission of human immunodeficiency virus type 1 mediated by immature and mature dendritic cells.J Virol. 2007 Sep;81(17):8933-43. doi: 10.1128/JVI.00878-07. Epub 2007 Jun 13. J Virol. 2007. PMID: 17567699 Free PMC article.
-
Tetherin does not significantly restrict dendritic cell-mediated HIV-1 transmission and its expression is upregulated by newly synthesized HIV-1 Nef.Retrovirology. 2011 Apr 19;8:26. doi: 10.1186/1742-4690-8-26. Retrovirology. 2011. PMID: 21504576 Free PMC article.
-
Cell-Free versus Cell-to-Cell Infection by Human Immunodeficiency Virus Type 1 and Human T-Lymphotropic Virus Type 1: Exploring the Link among Viral Source, Viral Trafficking, and Viral Replication.J Virol. 2016 Aug 12;90(17):7607-17. doi: 10.1128/JVI.00407-16. Print 2016 Sep 1. J Virol. 2016. PMID: 27334587 Free PMC article. Review.
-
HIV and mature dendritic cells: Trojan exosomes riding the Trojan horse?PLoS Pathog. 2010 Mar 26;6(3):e1000740. doi: 10.1371/journal.ppat.1000740. PLoS Pathog. 2010. PMID: 20360840 Free PMC article. Review.
Cited by
-
Development of human dendritic cells and their role in HIV infection: antiviral immunity versus HIV transmission.Front Microbiol. 2013 Jul 9;4:178. doi: 10.3389/fmicb.2013.00178. eCollection 2013. Front Microbiol. 2013. PMID: 23847602 Free PMC article.
-
Intercellular adhesion molecule 1 (ICAM-1), but not ICAM-2 and -3, is important for dendritic cell-mediated human immunodeficiency virus type 1 transmission.J Virol. 2009 May;83(9):4195-204. doi: 10.1128/JVI.00006-09. Epub 2009 Feb 11. J Virol. 2009. PMID: 19211748 Free PMC article.
-
Immunological synapse: structures, molecular mechanisms and therapeutic implications in disease.Signal Transduct Target Ther. 2025 Aug 11;10(1):254. doi: 10.1038/s41392-025-02332-6. Signal Transduct Target Ther. 2025. PMID: 40784895 Free PMC article. Review.
-
Cellular and Biochemical Mechanisms of the Retroviral Restriction Factor SAMHD1.ISRN Biochem. 2013 Jul 7;2013:728392. doi: 10.1155/2013/728392. ISRN Biochem. 2013. PMID: 24523981 Free PMC article.
-
HIV-1 interactions with cells: from viral binding to cell-cell transmission.Curr Protoc Cell Biol. 2009 Jun;Chapter 26:Unit 26.5. doi: 10.1002/0471143030.cb2605s43. Curr Protoc Cell Biol. 2009. PMID: 19499507 Free PMC article.
References
-
- Ardeshna, K. M., A. R. Pizzey, S. Devereux, and A. Khwaja. 2000. The PI3 kinase, p38 SAP kinase, and NF-kappaB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood 96:1039-1046. - PubMed
-
- Bakri, Y., C. Schiffer, V. Zennou, P. Charneau, E. Kahn, A. Benjouad, J. C. Gluckman, and B. Canque. 2001. The maturation of dendritic cells results in postintegration inhibition of HIV-1 replication. J. Immunol. 166:3780-3788. - PubMed
-
- Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

