Adrenergic modulation of olfactory bulb circuitry affects odor discrimination
- PMID: 17686948
- PMCID: PMC1951793
- DOI: 10.1101/lm.606407
Adrenergic modulation of olfactory bulb circuitry affects odor discrimination
Abstract
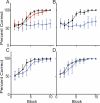
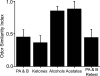
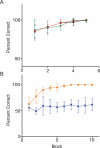
A rodent's survival depends upon its ability to perceive odor cues necessary to guide mate selection, sexual behavior, foraging, territorial formation, and predator avoidance. Arguably, the need to discriminate odor cues in a complex olfactory environment requires a highly adaptable olfactory system. Indeed, it has been proposed that context-dependent modulation of the initial sensory relay could alter olfactory perception. Interestingly, 40% of the adrenergic innervation from the locus coeruleus, fibers that are activated by contextual cues, innervates the first relay station in the olfactory system (the main olfactory bulb). Here we utilize restricted pharmacological inhibition of olfactory bulb noradrenergic receptors in awake-behaving animals. We show that combined blockade of alpha and beta adrenergic receptors does not impair two-odor discrimination behavior per se but does impair the ability to discriminate perceptually similar odors. Thus, contextual cues conveyed by noradrenergic fibers alter processing before the second synapse in the olfactory cortex, resulting in tuning of the ability to discriminate between similar odors.
Figures





References
-
- Abraham N.M., Spors H., Carleton A., Margrie T.W., Kuner T., Schaefer A.T. Maintaining accuracy at the expense of speed: Stimulus similarity defines odor discrimination time in mice. Neuron. 2004;44:865–876. - PubMed
-
- Berridge C.W., Waterhouse B.D. The locus coeruleus–noradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Res. Brain Res. Rev. 2003;42:33–84. - PubMed
-
- Bouret S., Sara S.J. Locus coeruleus activation modulates firing rate and temporal organization of odour-induced single-cell responses in rat piriform cortex. Eur. J. Neurosci. 2002;16:2371–2382. - PubMed
-
- Bouret S., Sara S.J. Reward expectation, orientation of attention and locus coeruleus–medial frontal cortex interplay during learning. Eur. J. Neurosci. 2004;20:791–802. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
