Expression of dominant-negative Dmp53 in the adult fly brain inhibits insulin signaling
- PMID: 17686972
- PMCID: PMC1948898
- DOI: 10.1073/pnas.0706121104
Expression of dominant-negative Dmp53 in the adult fly brain inhibits insulin signaling
Abstract
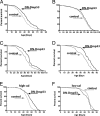
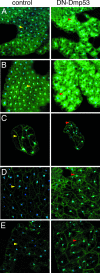
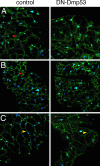
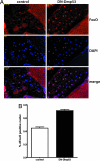
In Drosophila melanogaster, p53 (Dmp53) is an important mediator of longevity. Expression of dominant-negative (DN) forms of Dmp53 in adult neurons, but not in muscle or fat body cells, extends lifespan. The lifespan of calorie-restricted flies is not further extended by simultaneously expressing DN-Dmp53 in the nervous system, indicating that a decrease in Dmp53 activity may be a part of the CR lifespan-extending pathway in flies. In this report, we show that selective expression of DN-Dmp53 in only the 14 insulin-producing cells (IPCs) in the brain extends lifespan to the same extent as expression in all neurons and this lifespan extension is not additive with CR. DN-Dmp53-dependent lifespan extension is accompanied by reduction of Drosophila insulin-like peptide 2 (dILP2) mRNA levels and reduced insulin signaling (IIS) in the fat body, which suggests that Dmp53 may affect lifespan by modulating insulin signaling in the fly.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Masoro EJ. Sci Aging Knowledge Environ. 2003:RE2. - PubMed
-
- Tatar M, Bartke A, Antebi A. Science. 2003;299:1346–1351. - PubMed
-
- Goberdhan DC, Wilson C. Differentiation. 2003;71:375–397. - PubMed
-
- Obsil T, Ghirlando R, Anderson DE, Hickman AB, Dyda F. Biochemistry. 2003;42:15264–15272. - PubMed
-
- Wang Y, Oh SW, Deplancke B, Luo J, Walhout AJ, Tissenbaum HA. Mech Ageing Dev. 2006;127:741–747. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

