A lymphocytic choriomeningitis virus glycoprotein variant that is retained in the endoplasmic reticulum efficiently cross-primes CD8(+) T cell responses
- PMID: 17686978
- PMCID: PMC1948914
- DOI: 10.1073/pnas.0704423104
A lymphocytic choriomeningitis virus glycoprotein variant that is retained in the endoplasmic reticulum efficiently cross-primes CD8(+) T cell responses
Abstract
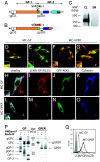
Recent studies indicate that T cell cross-priming preferentially occurs against long-lived, stable proteins. We have studied cross-priming by using the glycoprotein (GP) of lymphocytic choriomeningitis virus (LCMV), a protein that normally is not MHC class I cross-presented. This study shows that a C-terminally truncated, noncleavable variant of LCMV-GP led to the accumulation of stable, soluble GP trimers in the endoplasmic reticulum (ER) of the antigen donor cell, and thereby converted LCMV-GP into a potent immunogen for cytotoxic T lymphocyte cross-priming. Immunization of mice with tumor cells expressing an ER-retained LCMV-GP variant cross-primed protective antiviral cytotoxic T lymphocyte responses in vivo at least 10,000-fold better than immunization with cells expressing the cross-presentation-"resistant" wild-type LCMV-GP. Thus the ER is a cellular compartment that can provide antigen for cross-presentation, and modifications affecting stability and subcellular localization of the antigen significantly increase its availability for MHC class I cross-presentation. These findings impinge on vaccine strategies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
[Viral antigen induced autoimmunity: an animal model for diabetes mellitus type I].Verh Dtsch Ges Pathol. 1994;78:177-9. Verh Dtsch Ges Pathol. 1994. PMID: 7533982 German.
-
Anti-viral protection and prevention of lymphocytic choriomeningitis or of the local footpad swelling reaction in mice by immunization with vaccinia-recombinant virus expressing LCMV-WE nucleoprotein or glycoprotein.Eur J Immunol. 1989 Mar;19(3):417-24. doi: 10.1002/eji.1830190302. Eur J Immunol. 1989. PMID: 2468501
-
Cross-priming of a single viral protein from lymphocytic choriomeningitis virus alters immunodominance hierarchies of CD8+ T cells during subsequent viral infections.Viral Immunol. 2007 Dec;20(4):585-98. doi: 10.1089/vim.2007.0062. Viral Immunol. 2007. PMID: 18158732
-
Using recombinant coxsackievirus B3 to evaluate the induction and protective efficacy of CD8+ T cells during picornavirus infection.J Virol. 2001 Mar;75(5):2377-87. doi: 10.1128/JVI.75.5.2377-2387.2001. J Virol. 2001. PMID: 11160741 Free PMC article.
-
Vaccination with an adenoviral vector encoding the tumor antigen directly linked to invariant chain induces potent CD4(+) T-cell-independent CD8(+) T-cell-mediated tumor control.Eur J Immunol. 2009 Oct;39(10):2725-36. doi: 10.1002/eji.200939543. Eur J Immunol. 2009. PMID: 19637230
Cited by
-
Alphavirus vector-based replicon particles expressing multivalent cross-protective Lassa virus glycoproteins.Vaccine. 2018 Jan 29;36(5):683-690. doi: 10.1016/j.vaccine.2017.12.046. Epub 2017 Dec 26. Vaccine. 2018. PMID: 29287681 Free PMC article.
-
CD169+ macrophages are sufficient for priming of CTLs with specificities left out by cross-priming dendritic cells.Proc Natl Acad Sci U S A. 2015 Apr 28;112(17):5461-6. doi: 10.1073/pnas.1423356112. Epub 2015 Apr 14. Proc Natl Acad Sci U S A. 2015. PMID: 25922518 Free PMC article.
-
Antigen design enhances the immunogenicity of Semliki Forest virus-based therapeutic human papillomavirus vaccines.Gene Ther. 2015 Jul;22(7):560-7. doi: 10.1038/gt.2015.24. Epub 2015 Mar 26. Gene Ther. 2015. PMID: 25756550
-
A polymeric protein induces specific cytotoxicity in a TLR4 dependent manner in the absence of adjuvants.PLoS One. 2012;7(9):e45705. doi: 10.1371/journal.pone.0045705. Epub 2012 Sep 24. PLoS One. 2012. PMID: 23029192 Free PMC article.
-
The viral context instructs the redundancy of costimulatory pathways in driving CD8(+) T cell expansion.Elife. 2015 Aug 11;4:e07486. doi: 10.7554/eLife.07486. Elife. 2015. PMID: 26263500 Free PMC article.
References
-
- Rock KL, Shen L. Immunol Rev. 2005;207:166–183. - PubMed
-
- Heath WR, Belz GT, Behrens GM, Smith CM, Forehan SP, Parish IA, Davey GM, Wilson NS, Carbone FR, Villadangos JA. Immunol Rev. 2004;199:9–26. - PubMed
-
- den Haan JM, Bevan MJ. Curr Opin Immunol. 2001;13:437–441. - PubMed
-
- Norbury CC, Hewlett LJ, Prescott AR, Shastri N, Watts C. Immunity. 1995;3:783–791. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

