Engineering metal ion coordination to regulate amyloid fibril assembly and toxicity
- PMID: 17686982
- PMCID: PMC1948904
- DOI: 10.1073/pnas.0702669104
Engineering metal ion coordination to regulate amyloid fibril assembly and toxicity
Abstract
Protein and peptide assembly into amyloid has been implicated in functions that range from beneficial epigenetic controls to pathological etiologies. However, the exact structures of the assemblies that regulate biological activity remain poorly defined. We have previously used Zn(2+) to modulate the assembly kinetics and morphology of congeners of the amyloid beta peptide (Abeta) associated with Alzheimer's disease. We now reveal a correlation among Abeta-Cu(2+) coordination, peptide self-assembly, and neuronal viability. By using the central segment of Abeta, HHQKLVFFA or Abeta(13-21), which contains residues H13 and H14 implicated in Abeta-metal ion binding, we show that Cu(2+) forms complexes with Abeta(13-21) and its K16A mutant and that the complexes, which do not self-assemble into fibrils, have structures similar to those found for the human prion protein, PrP. N-terminal acetylation and H14A substitution, Ac-Abeta(13-21)H14A, alters metal coordination, allowing Cu(2+) to accelerate assembly into neurotoxic fibrils. These results establish that the N-terminal region of Abeta can access different metal-ion-coordination environments and that different complexes can lead to profound changes in Abeta self-assembly kinetics, morphology, and toxicity. Related metal-ion coordination may be critical to the etiology of other neurodegenerative diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


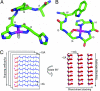
 O was introduced into the peptide at Phe-20; L17, 13C
O was introduced into the peptide at Phe-20; L17, 13C O was introduced into the peptide at Leu-17.
O was introduced into the peptide at Leu-17.

References
-
- Carrell RW, Lomas DA. Lancet. 1997;350:134–138. - PubMed
-
- Patino MM, Liu JJ, Glover JR, Lindquist S. Science. 1996;273:622–626. - PubMed
-
- Si K, Giustetto M, Etkin A, Hsu R, Janisiewicz AM, Miniaci MC, Kim JH, Zhu H, Kandel ER. Cell. 2003;115:893–904. - PubMed
-
- Si K, Lindquist S, Kandel ER. Cell. 2003;115:879–891. - PubMed
-
- Dobson CM. Nature. 2003;426:884–890. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

