Plasticity of polyubiquitin recognition as lysosomal targeting signals by the endosomal sorting machinery
- PMID: 17686993
- PMCID: PMC1995726
- DOI: 10.1091/mbc.e07-07-0678
Plasticity of polyubiquitin recognition as lysosomal targeting signals by the endosomal sorting machinery
Abstract
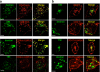
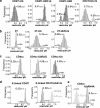
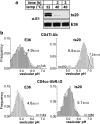
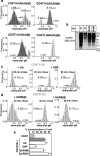
Lysosomal targeting is fundamental for the regulated disposal of ubiquitinated membrane proteins from the cell surface. To elucidate ubiquitin (Ub) configurations that are necessary and sufficient as multivesicular body (MVB)/lysosomal-sorting motifs, the intraendosomal destination and transport kinetics of model transmembrane cargo molecules bearing monoubiquitinated, multi-monoubiquitinated, or polyubiquitinated cytoplasmic tails were determined. Monomeric CD4 chimeras with K63-linked poly-Ub chains and tetrameric CD4-mono-Ub chimeras were rapidly targeted to the lysosome. In contrast, lysosomal delivery of CD4 chimeras exposing K48-linked Ub chains was delayed, whereas delivery of monoubiquitinated CD4 chimeras was undetectable. Similar difference was observed in the lysosomal targeting of mono- versus polyubiquitinated invariant chain and CD4 ubiquitinated by the MARCH (membrane-associated RING-CH) IV Ub ligase. Consistent with this, Hrs (hepatocyte growth factor regulated tyrosine kinase phosphorylated substrate), an endosomal sorting adaptor, binds preferentially to K63-Ub chain and negligibly to mono-Ub. These results highlight the plasticity of Ub as a sorting signal and its recognition by the endosomal sorting machinery, and together with previous data, suggest a regulatory role for assembly and disassembly of Ub chains of specific topology in lysosomal cargo sorting.
Figures





References
-
- Amerik A. Y., Hochstrasser M. Mechanism and function of deubiquitinating enzymes. Biochim. Biophys. Acta. 2004;1695:189–207. - PubMed
-
- Bache K. G., Raiborg C., Mehlum A., Stenmark H. STAM and Hrs are subunits of a multivalent ubiquitin-binding complex on early endosomes. J. Biol. Chem. 2003;278:12513–12521. - PubMed
-
- Barriere H., Nemes C., Lechardeur D., Khan-Mohammad M., Fruh K., Lukacs G. L. Molecular basis of oligoubiquitin-dependent internalization of membrane proteins in mammalian cells. Traffic. 2006;7:282–297. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

