Role of calcineurin in nicotine-mediated locomotor sensitization
- PMID: 17687035
- PMCID: PMC6672935
- DOI: 10.1523/JNEUROSCI.2601-07.2007
Role of calcineurin in nicotine-mediated locomotor sensitization
Abstract
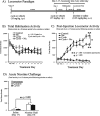
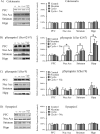
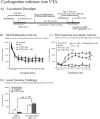
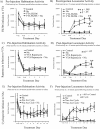
Calcineurin is a serine/threonine phosphatase that contributes to the effects of nicotine on calcium signaling in cultured cortical neurons; however, the role of calcineurin in behavioral responses to nicotine in vivo has not been examined. We therefore determined whether calcineurin blockade could alter nicotine-mediated locomotor sensitization in Sprague Dawley rats using systemic or brain region-specific administration of the calcineurin inhibitors cyclosporine or FK506. Systemic cyclosporine administration decreased calcineurin activity in the brain, attenuated nicotine-mediated locomotor sensitization, and blocked the effects of nicotine on DARPP32 (dopamine- and cAMP-regulated phosphoprotein-32) activation in the striatum. Direct infusion of calcineurin inhibitors cyclosporine or FK506 into the ventral tegmental area (VTA) also attenuated nicotine-mediated locomotor sensitization, whereas infusion of rapamycin, which binds to FK-binding protein but does not inhibit calcineurin, did not affect sensitization. Together, the data suggest that activation of calcineurin, particularly in the VTA, is a novel signaling event important for nicotine-mediated behavior and intracellular signaling.
Figures

References
-
- Bahler M, Greengard P. Synapsin I bundles F-actin in a phosphorylation-dependent manner. Nature. 1987;326:704–707. - PubMed
-
- Biala G. Calcium channel antagonists suppress nicotine-induced place preference and locomotor sensitization in rodents. Pol J Pharmacol. 2003;55:327–335. - PubMed
-
- Brunzell D, Russell D, Picciotto M. In vivo nicotine treatment regulates mesocorticolimbic CREB and ERK signaling in C57Bl/6J mice. J Neurochem. 2003;84:1431–1441. - PubMed
-
- Centers for Disease Control and Prevention. Tobacco use among adults—United States 2005. MMWR Morb Mortal Wkly Rep. 2006;55:1145–1148. - PubMed
-
- Chen TC, Law B, Kondratyuk T, Rossie S. Identification of soluble protein phosphatases that dephosphorylate voltage-sensitive sodium channels in rat brain. J Biol Chem. 1995;270:7750–7756. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F31 DA021459/DA/NIDA NIH HHS/United States
- P50 AA015632/AA/NIAAA NIH HHS/United States
- K02 DA000436/DA/NIDA NIH HHS/United States
- T32 MH018882/MH/NIMH NIH HHS/United States
- DA11717/DA/NIDA NIH HHS/United States
- R37 DA014241/DA/NIDA NIH HHS/United States
- AA15632/AA/NIAAA NIH HHS/United States
- MH18882/MH/NIMH NIH HHS/United States
- DA00436/DA/NIDA NIH HHS/United States
- R01 DA014241/DA/NIDA NIH HHS/United States
- DA021459/DA/NIDA NIH HHS/United States
- DA14241/DA/NIDA NIH HHS/United States
- R01 DA011717/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
