Kinetic properties of Cl uptake mediated by Na+-dependent K+-2Cl cotransport in immature rat neocortical neurons
- PMID: 17687039
- PMCID: PMC6672936
- DOI: 10.1523/JNEUROSCI.5041-06.2007
Kinetic properties of Cl uptake mediated by Na+-dependent K+-2Cl cotransport in immature rat neocortical neurons
Abstract
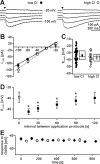
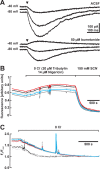
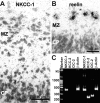
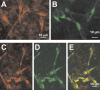
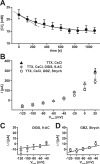
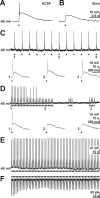
GABA, the main inhibitory neurotransmitter in the adult nervous system, evokes depolarizing membrane responses in immature neurons, which are crucial for the generation of early network activity. Although it is well accepted that depolarizing GABA actions are caused by an elevated intracellular Cl- concentration ([Cl-]i), the mechanisms of Cl- accumulation in immature neurons are still a matter of debate. Using patch-clamp, microfluorimetric, immunohistochemical, and molecular biological approaches, we studied the mechanism of Cl- uptake in Cajal-Retzius (CR) cells of immature [postnatal day 0 (P0) to P3] rat neocortex. Gramicidin-perforated patch-clamp and 6-methoxy-N-ethylquinolinium-microfluorimetric measurements revealed a steady-state [Cl-]i of approximately 30 mM that was reduced to values close to passive distribution by bumetanide or Na+-free solutions, suggesting a participation of Na+-K+-2Cl- cotransport isoform 1 (NKCC1) in maintaining elevated [Cl-]i. Expression of NKCC1 was found in CR cells on the mRNA and protein levels. To determine the contribution of NKCC1 to [Cl-]i homeostasis in detail, Cl- uptake rates were analyzed after artificial [Cl-]i depletion. Active Cl- uptake was relatively slow (47.2 +/- 5.0 microM/s) and was abolished by bumetanide or Na+-free solution. Accordingly, whole-cell patch-clamp recordings revealed a low Cl- conductance in CR cells. The low capacity of NKCC1-mediated Cl- uptake was sufficient to maintain excitatory GABAergic membrane responses, however, only at low stimulation frequencies. In summary, our results demonstrate that NKCC1 is abundant in CR cells of immature rat neocortex and that the slow Cl- uptake mediated by this transporter is sufficient to maintain high [Cl-]i required to render GABA responses excitatory.
Figures




References
-
- Achilles K, Okabe A, Ikeda M, Shimizu-Okabe C, Fukuda A, Luhmann HJ, Kilb W. Excitatory GABA actions in Cajal-Retzius cells are mediated by NKCC1. FENS Abstr. 2006;3:A1118–1.
-
- Aronson PS. Kinetic properties of the plasma membrane Na+-H+ exchanger. Annu Rev Physiol. 1985;47:545–560. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
