Local and global effects of I(h) distribution in dendrites of mammalian neurons
- PMID: 17687042
- PMCID: PMC6672943
- DOI: 10.1523/JNEUROSCI.5284-06.2007
Local and global effects of I(h) distribution in dendrites of mammalian neurons
Abstract
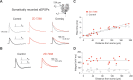
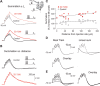
The hyperpolarization-activated cation current I(h) exhibits a steep gradient of channel density in dendrites of pyramidal neurons, which is associated with location independence of temporal summation of EPSPs at the soma. In striking contrast, here we show by using dendritic patch-clamp recordings that in cerebellar Purkinje cells, the principal neurons of the cerebellar cortex, I(h) exhibits a uniform dendritic density, while location independence of EPSP summation is observed. Using compartmental modeling in realistic and simplified dendritic geometries, we demonstrate that the dendritic distribution of I(h) only weakly affects the degree of temporal summation at the soma, while having an impact at the dendritic input location. We further analyze the effect of I(h) on temporal summation using cable theory and derive bounds for temporal summation for any spatial distribution of I(h). We show that the total number of I(h) channels, not their distribution, governs the degree of temporal summation of EPSPs. Our findings explain the effect of I(h) on EPSP shape and temporal summation, and suggest that neurons are provided with two independent degrees of freedom for different functions: the total amount of I(h) (controlling the degree of temporal summation of dendritic inputs at the soma) and the dendritic spatial distribution of I(h) (regulating local dendritic processing).
Figures





References
-
- Berger T, Larkum ME, Lüscher HR. High Ih channel density in the distal apical dendrite of layer V pyramidal cells increases bidirectional attenuation of EPSPs. J Neurophysiol. 2001;85:855–868. - PubMed
-
- Berger T, Senn W, Lüscher HR. Hyperpolarization-activated current Ih disconnects somatic and dendritic spike initiation zones in layer V pyramidal neurons. J Neurophysiol. 2003;90:2428–2437. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
