Munc18-1: sequential interactions with the fusion machinery stimulate vesicle docking and priming
- PMID: 17687045
- PMCID: PMC6672934
- DOI: 10.1523/JNEUROSCI.0658-07.2007
Munc18-1: sequential interactions with the fusion machinery stimulate vesicle docking and priming
Abstract
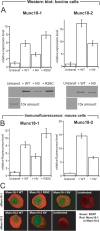
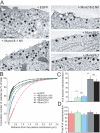
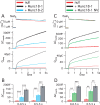
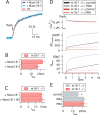
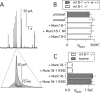
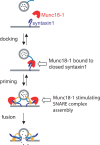
Exocytosis of secretory or synaptic vesicles is executed by a mechanism including the SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) proteins. Munc18-1 is a part of this fusion machinery, but its role is controversial because it is indispensable for fusion but also inhibits the assembly of purified SNAREs in vitro. This inhibition reflects the binding of Munc18-1 to a closed conformation of the target-SNARE syntaxin1. The controversy would be solved if binding to closed syntaxin1 were shown to be stimulatory for vesicle fusion and/or additional essential interactions were identified between Munc18-1 and the fusion machinery. Here, we provide evidence for both notions by dissecting sequential steps of the exocytotic cascade while expressing Munc18 variants in the Munc18-1 null background. In Munc18-1 null chromaffin cells, vesicle docking is abolished and syntaxin levels are reduced. A mutation that diminished Munc18 binding to syntaxin1 in vitro attenuated the vesicle-docking step but rescued vesicle priming in excess of docking. Conversely, expressing the Munc18-2 isoform, which also displays binding to closed syntaxin1, rescued vesicle docking identical with Munc18-1 but impaired more downstream vesicle priming steps. All Munc18 variants restored syntaxin1 levels at least to wild-type levels, showing that the docking phenotype is not caused by syntaxin1 reduction. None of the Munc18 variants affected vesicle fusion kinetics or fusion pore duration. In conclusion, binding of Munc18-1 to closed syntaxin1 stimulates vesicle docking and a distinct interaction mode regulates the consecutive priming step.
Figures



Similar articles
-
Extension of Helix 12 in Munc18-1 Induces Vesicle Priming.J Neurosci. 2016 Jun 29;36(26):6881-91. doi: 10.1523/JNEUROSCI.0007-16.2016. J Neurosci. 2016. PMID: 27358447 Free PMC article.
-
SNAREpin assembly by Munc18-1 requires previous vesicle docking by synaptotagmin 1.J Biol Chem. 2012 Sep 7;287(37):31041-9. doi: 10.1074/jbc.M112.386805. Epub 2012 Jul 18. J Biol Chem. 2012. PMID: 22810233 Free PMC article.
-
MUNC18-1 regulates the submembrane F-actin network, independently of syntaxin1 targeting, via hydrophobicity in β-sheet 10.J Cell Sci. 2019 Dec 2;132(23):jcs234674. doi: 10.1242/jcs.234674. J Cell Sci. 2019. PMID: 31719162
-
Neuronal SNARE complex assembly guided by Munc18-1 and Munc13-1.FEBS Open Bio. 2022 Nov;12(11):1939-1957. doi: 10.1002/2211-5463.13394. Epub 2022 Mar 22. FEBS Open Bio. 2022. PMID: 35278279 Free PMC article. Review.
-
Conflicting views on the membrane fusion machinery and the fusion pore.Annu Rev Cell Dev Biol. 2009;25:513-37. doi: 10.1146/annurev.cellbio.24.110707.175239. Annu Rev Cell Dev Biol. 2009. PMID: 19575641 Review.
Cited by
-
Doc2b serves as a scaffolding platform for concurrent binding of multiple Munc18 isoforms in pancreatic islet β-cells.Biochem J. 2014 Dec 1;464(2):251-8. doi: 10.1042/BJ20140845. Biochem J. 2014. PMID: 25190515 Free PMC article.
-
G protein betagamma subunits modulate the number and nature of exocytotic fusion events in adrenal chromaffin cells independent of calcium entry.J Neurophysiol. 2008 Nov;100(5):2929-39. doi: 10.1152/jn.90839.2008. Epub 2008 Sep 24. J Neurophysiol. 2008. PMID: 18815342 Free PMC article.
-
Platelet granule exocytosis: a comparison with chromaffin cells.Front Endocrinol (Lausanne). 2013 Jun 26;4:77. doi: 10.3389/fendo.2013.00077. eCollection 2013. Front Endocrinol (Lausanne). 2013. PMID: 23805129 Free PMC article.
-
Morphological docking of secretory vesicles.Histochem Cell Biol. 2010 Aug;134(2):103-13. doi: 10.1007/s00418-010-0719-5. Epub 2010 Jun 26. Histochem Cell Biol. 2010. PMID: 20577884 Free PMC article. Review.
-
Vesicle pools: lessons from adrenal chromaffin cells.Front Synaptic Neurosci. 2011 Feb 1;3:2. doi: 10.3389/fnsyn.2011.00002. eCollection 2011. Front Synaptic Neurosci. 2011. PMID: 21423410 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
