Dendritic HCN2 channels constrain glutamate-driven excitability in reticular thalamic neurons
- PMID: 17687049
- PMCID: PMC6672930
- DOI: 10.1523/JNEUROSCI.1630-07.2007
Dendritic HCN2 channels constrain glutamate-driven excitability in reticular thalamic neurons
Abstract
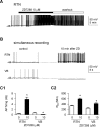
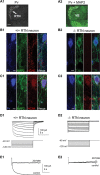
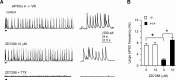
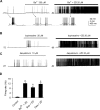
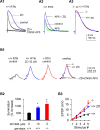
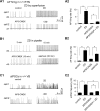
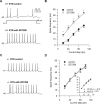
Hyperpolarization activated cyclic nucleotide (HCN) gated channels conduct a current, I(h); how I(h) influences excitability and spike firing depends primarily on channel distribution in subcellular compartments. For example, dendritic expression of HCN1 normalizes somatic voltage responses and spike output in hippocampal and cortical neurons. We reported previously that HCN2 is predominantly expressed in dendritic spines in reticular thalamic nucleus (RTN) neurons, but the functional impact of such nonsomatic HCN2 expression remains unknown. We examined the role of HCN2 expression in regulating RTN excitability and GABAergic output from RTN to thalamocortical relay neurons using wild-type and HCN2 knock-out mice. Pharmacological blockade of I(h) significantly increased spike firing in RTN neurons and large spontaneous IPSC frequency in relay neurons; conversely, pharmacological enhancement of HCN channel function decreased spontaneous IPSC frequency. HCN2 deletion abolished I(h) in RTN neurons and significantly decreased sensitivity to 8-bromo-cAMP and lamotrigine. Recapitulating the effects of I(h) block, HCN2 deletion increased both temporal summation of EPSPs in RTN neurons as well as GABAergic output to postsynaptic relay neurons. The enhanced excitability of RTN neurons after I(h) block required activation of ionotropic glutamate receptors; consistent with this was the colocalization of HCN2 and glutamate receptor 4 subunit immunoreactivities in dendritic spines of RTN neurons. The results indicate that, in mouse RTN neurons, HCN2 is the primary functional isoform underlying I(h) and expression of HCN2 constrains excitatory synaptic integration.
Figures



Similar articles
-
Compartmental distribution of hyperpolarization-activated cyclic-nucleotide-gated channel 2 and hyperpolarization-activated cyclic-nucleotide-gated channel 4 in thalamic reticular and thalamocortical relay neurons.Neuroscience. 2006 Sep 15;141(4):1811-25. doi: 10.1016/j.neuroscience.2006.05.034. Epub 2006 Jun 27. Neuroscience. 2006. PMID: 16806719
-
Selective participation of somatodendritic HCN channels in inhibitory but not excitatory synaptic integration in neurons of the subthalamic nucleus.J Neurosci. 2010 Nov 24;30(47):16025-40. doi: 10.1523/JNEUROSCI.3898-10.2010. J Neurosci. 2010. PMID: 21106841 Free PMC article.
-
Targeted deletion of Kcne2 impairs HCN channel function in mouse thalamocortical circuits.PLoS One. 2012;7(8):e42756. doi: 10.1371/journal.pone.0042756. Epub 2012 Aug 3. PLoS One. 2012. PMID: 22880098 Free PMC article.
-
HCN channels and absence seizures.Neurobiol Dis. 2023 Jun 1;181:106107. doi: 10.1016/j.nbd.2023.106107. Epub 2023 Mar 30. Neurobiol Dis. 2023. PMID: 37001612 Review.
-
Neurophysiology of HCN channels: from cellular functions to multiple regulations.Prog Neurobiol. 2014 Jan;112:1-23. doi: 10.1016/j.pneurobio.2013.10.001. Epub 2013 Oct 29. Prog Neurobiol. 2014. PMID: 24184323 Review.
Cited by
-
Volatile anesthetics inhibit presynaptic cGMP signaling to depress presynaptic excitability in rat hippocampal neurons.Neuropharmacology. 2023 Dec 1;240:109705. doi: 10.1016/j.neuropharm.2023.109705. Epub 2023 Sep 6. Neuropharmacology. 2023. PMID: 37683886 Free PMC article.
-
Retinoic Acid-Mediated Regulation of GLI3 Enables Efficient Motoneuron Derivation from Human ESCs in the Absence of Extrinsic SHH Activation.J Neurosci. 2015 Aug 19;35(33):11462-81. doi: 10.1523/JNEUROSCI.3046-14.2015. J Neurosci. 2015. PMID: 26290227 Free PMC article.
-
Blunted diurnal firing in lateral habenula projections to dorsal raphe nucleus and delayed photoentrainment in stress-susceptible mice.PLoS Biol. 2021 Mar 10;19(3):e3000709. doi: 10.1371/journal.pbio.3000709. eCollection 2021 Mar. PLoS Biol. 2021. PMID: 33690628 Free PMC article.
-
General anesthesia mediated by effects on ion channels.World J Crit Care Med. 2012 Jun 4;1(3):80-93. doi: 10.5492/wjccm.v1.i3.80. eCollection 2012 Jun 4. World J Crit Care Med. 2012. PMID: 24701405 Free PMC article. Review.
-
Regulation of epileptiform discharges in rat neocortex by HCN channels.J Neurophysiol. 2013 Oct;110(8):1733-43. doi: 10.1152/jn.00955.2012. Epub 2013 Jul 17. J Neurophysiol. 2013. PMID: 23864381 Free PMC article.
References
-
- Abbas SY, Ying SW, Goldstein PA. Compartmental distribution of hyperpolarization-activated cyclic-nucleotide-gated channel 2 and hyperpolarization-activated cyclic-nucleotide-gated channel 4 in thalamic reticular and thalamocortical relay neurons. Neuroscience. 2006;141:1811–1825. - PubMed
-
- Arcelli P, Frassoni C, Regondi MC, De Biasi S, Spreafico R. GABAergic neurons in mammalian thalamus: a marker of thalamic complexity? Brain Res Bull. 1997;42:27–37. - PubMed
-
- Berger T, Lüscher HR. Associative somatodendritic interaction in layer V pyramidal neurons is not affected by the antiepileptic drug lamotrigine. Eur J Neurosci. 2004;20:1688–1693. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
