Mitogenic component in polar lipid-enriched Anaplasma phagocytophilum membranes
- PMID: 17687112
- PMCID: PMC2168108
- DOI: 10.1128/CVI.00204-07
Mitogenic component in polar lipid-enriched Anaplasma phagocytophilum membranes
Abstract
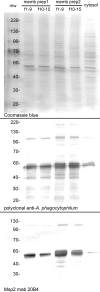
Human granulocytic anaplasmosis is an emerging tick-borne disease caused by Anaplasma phagocytophilum. A. phagocytophilum cells activate Toll-like receptor 2 signaling and possess mitogenic activity, and A. phagocytophilum infection in vivo activates NKT cells unrelated to major surface protein 2 (Msp2) hypervariable region expression. Thus, we hypothesized that lipoprotein or glycolipid components of A. phagocytophilum membranes could be important triggers of the innate immune response and immunopathology. A. phagocytophilum membranes depleted of Msp2 and protein antigens enhanced the proliferation of naïve mouse splenocytes beyond that of untreated membranes. Protein-depleted and polar lipid-enriched membranes from low-passage A. phagocytophilum cultures enhanced naïve splenocyte lymphoproliferation to a much greater degree than did these fractions from high-passage cultures of bacterial membranes (1.8- to 3.7-fold for protein-depleted fractions and 4.8- to > or =17.7-fold for polar lipid-enriched fractions). These results support the hypothesis that components that are enriched among polar lipids in the A. phagocytophilum membrane stimulate innate immune cell proliferation, possibly activating NKT cells that link innate and adaptive immunity, and immunopathology.
Figures

Similar articles
-
Differential innate immune cell activation and proinflammatory response in Anaplasma phagocytophilum infection.Infect Immun. 2007 Jun;75(6):3124-30. doi: 10.1128/IAI.00098-07. Epub 2007 Apr 2. Infect Immun. 2007. PMID: 17403880 Free PMC article.
-
Msp2 variation in Anaplasma phagocytophilum in vivo does not stimulate T cell immune responses or interferon-gamma production.FEMS Immunol Med Microbiol. 2007 Apr;49(3):374-86. doi: 10.1111/j.1574-695X.2007.00214.x. Epub 2007 Feb 7. FEMS Immunol Med Microbiol. 2007. PMID: 17286796
-
Sequential analysis of Anaplasma phagocytophilum msp2 transcription in murine and equine models of human granulocytic anaplasmosis.Clin Vaccine Immunol. 2008 Mar;15(3):418-24. doi: 10.1128/CVI.00417-07. Epub 2007 Dec 19. Clin Vaccine Immunol. 2008. PMID: 18094110 Free PMC article.
-
The biological basis of severe outcomes in Anaplasma phagocytophilum infection.FEMS Immunol Med Microbiol. 2012 Feb;64(1):13-20. doi: 10.1111/j.1574-695X.2011.00909.x. Epub 2011 Dec 19. FEMS Immunol Med Microbiol. 2012. PMID: 22098465 Free PMC article. Review.
-
Immune evasion and immunosuppression by Anaplasma phagocytophilum, the causative agent of tick-borne fever of ruminants and human granulocytic anaplasmosis.Vet J. 2008 Jan;175(1):37-44. doi: 10.1016/j.tvjl.2006.11.019. Epub 2007 Feb 1. Vet J. 2008. PMID: 17275372 Review.
Cited by
-
Innate immunity in rickettsial infections.Front Cell Infect Microbiol. 2023 May 9;13:1187267. doi: 10.3389/fcimb.2023.1187267. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37228668 Free PMC article. Review.
-
Gene expression profile suggests that pigs (Sus scrofa) are susceptible to Anaplasma phagocytophilum but control infection.Parasit Vectors. 2012 Aug 30;5:181. doi: 10.1186/1756-3305-5-181. Parasit Vectors. 2012. PMID: 22935149 Free PMC article.
-
Anaplasma phagocytophilum-Related Defects in CD8, NKT, and NK Lymphocyte Cytotoxicity.Front Immunol. 2018 Apr 9;9:710. doi: 10.3389/fimmu.2018.00710. eCollection 2018. Front Immunol. 2018. PMID: 29686681 Free PMC article.
-
Anaplasma phagocytophilum: deceptively simple or simply deceptive?Future Microbiol. 2012 Jun;7(6):719-31. doi: 10.2217/fmb.12.45. Future Microbiol. 2012. PMID: 22702526 Free PMC article. Review.
-
Dexamethasone-induced cytokine changes associated with diminished disease severity in horses infected with Anaplasma phagocytophilum.Clin Vaccine Immunol. 2011 Nov;18(11):1962-8. doi: 10.1128/CVI.05034-11. Epub 2011 Aug 31. Clin Vaccine Immunol. 2011. PMID: 21880854 Free PMC article.
References
-
- Browning, M. D., J. W. Garyu, J. S. Dumler, and D. G. Scorpio. 2006. Role of reactive nitrogen species in development of hepatic injury in a C57bl/6 mouse model of human granulocytic anaplasmosis. Comp. Med. 56:55-62. - PubMed
-
- Bunnell, J. E., E. R. Trigiani, S. R. Srinivas, and J. S. Dumler. 1999. Development and distribution of pathologic lesions are related to immune status and tissue deposition of human granulocytic ehrlichiosis agent-infected cells in a murine model system. J. Infect. Dis. 180:546-550. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

