Cellular adaptations to hypoxia and acidosis during somatic evolution of breast cancer
- PMID: 17687336
- PMCID: PMC2360372
- DOI: 10.1038/sj.bjc.6603922
Cellular adaptations to hypoxia and acidosis during somatic evolution of breast cancer
Abstract
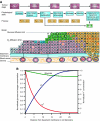
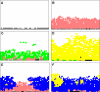
Conceptual models of carcinogenesis typically consist of an evolutionary sequence of heritable changes in genes controlling proliferation, apoptosis, and senescence. We propose that these steps are necessary but not sufficient to produce invasive breast cancer because intraductal tumour growth is also constrained by hypoxia and acidosis that develop as cells proliferate into the lumen and away from the underlying vessels. This requires evolution of glycolytic and acid-resistant phenotypes that, we hypothesise, is critical for emergence of invasive cancer. Mathematical models demonstrate severe hypoxia and acidosis in regions of intraductal tumours more than 100 microm from the basement membrane. Subsequent evolution of glycolytic and acid-resistant phenotypes leads to invasive proliferation. Multicellular spheroids recapitulating ductal carcinoma in situ (DCIS) microenvironmental conditions demonstrate upregulated glucose transporter 1 (GLUT1) as adaptation to hypoxia followed by growth into normoxic regions in qualitative agreement with model predictions. Clinical specimens of DCIS exhibit periluminal distribution of GLUT-1 and Na(+)/H(+) exchanger (NHE) indicating transcriptional activation by hypoxia and clusters of the same phenotype in the peripheral, presumably normoxic regions similar to the pattern predicted by the models and observed in spheroids. Upregulated GLUT-1 and NHE-1 were observed in microinvasive foci and adjacent intraductal cells. Adaptation to hypoxia and acidosis may represent key events in transition from in situ to invasive cancer.
Figures
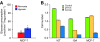



References
-
- Anderson ARA (2005) A hybrid mathematical model of solid tumour invasion: the importance of cell adhesion. Math Med Biol 22: 163–186 - PubMed
-
- Bischof G, Cosentini E, Hamilton G, Riegler M, Zacherl J, Teleky B, Feil W, Schiessel R, Machen TE, Wenzl E (1996) Effects of extracellular pH on intracellular pH-regulation and growth in a human colon carcinoma cell-line. Biochim Biophys Acta 1282: 131–139 - PubMed
-
- Brown RS, Goodman TM, Zasadny KE, Greenson JK, Wahl RL (2002) Expression of hexokinase II and Glut-1 in untreated human breast cancer. Nuclear Med and Biol 29: 443–453 - PubMed
-
- Brown RS, Leung JY, Fisher SJ, Frey KA, Ethier SP, Wahl RL (1996) Intratumoral distribution of tritiated-FDG in breast carcinoma: correlation between Glut-1 expression and FDG uptake. J Nuc Med 37: 1042–1047 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

