Heat stress inhibits skeletal muscle hypertrophy
- PMID: 17688192
- PMCID: PMC1949332
- DOI: 10.1379/csc-233r.1
Heat stress inhibits skeletal muscle hypertrophy
Abstract
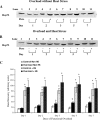
Heat shock proteins (Hsps) are molecular chaperones that aid in protein synthesis and trafficking and have been shown to protect cells/tissues from various protein damaging stressors. To determine the extent to which a single heat stress and the concurrent accumulation of Hsps influences the early events of skeletal muscle hypertrophy, Sprague-Dawley rats were heat stressed (42 degrees C, 15 minutes) 24 hours prior to overloading 1 plantaris muscle by surgical removal of the gastrocnemius muscle. The contralateral plantaris muscles served as controls. Heat-stressed and/or overloaded plantaris muscles were assessed for muscle mass, total muscle protein, muscle protein concentration, Type I myosin heavy chain (Type I MHC) content, as well as Hsp72 and Hsp25 content over the course of 7 days following removal of the gastrocnemius muscle. As expected, in non-heat-stressed animals, muscle mass, total muscle protein and MHC I content were significantly increased (P < 0.05) following overload. In addition, Hsp25 and Hsp72 increased significantly after 2 and 3 days of overload, respectively. A prior heat stress-elevated Hsp25 content to levels similar to those measured following overload alone, but heat stress-induced Hsp72 content was increased significantly greater than was elicited by overload alone. Moreover, overloaded muscles from animals that experienced a prior heat stress showed a lower muscle mass increase at 5 and 7 days; a reduced total muscle protein elevation at 3, 5, and 7 days; reduced protein concentration; and a diminished Type I MHC content accumulation at 3, 5, and 7 days relative to nonheat-stressed animals. These data suggest that a prior heat stress and/or the consequent accumulation of Hsps may inhibit increases in muscle mass, total muscle protein content, and Type I MHC in muscles undergoing hypertrophy.
Figures

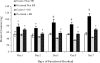

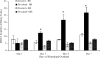
References
-
- Bischoff R 1994 The satellite cell and muscle regeneration. In: Myogenesis, ed Engel AG, Franszini-Armstrong C. McGraw-Hill, New York, 97–118.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
