Neuronal nicotinic receptor agonists for the treatment of attention-deficit/hyperactivity disorder: focus on cognition
- PMID: 17689498
- PMCID: PMC2974320
- DOI: 10.1016/j.bcp.2007.07.002
Neuronal nicotinic receptor agonists for the treatment of attention-deficit/hyperactivity disorder: focus on cognition
Abstract
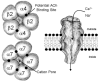
Attention deficit/hyperactivity disorder (ADHD) is the most commonly diagnosed neurobehavioral disorder in children and adolescents, and in about half of these patients, significant symptomology continues into adulthood. Although impulsivity and hyperactivity are the most salient features of ADHD, cognitive deficits, particularly impairments in attention and executive function, are an important component, particularly in adolescents and adults, with over 90% of adults seeking treatment for ADHD manifesting cognitive dysfunction. Currently available medications treat the core ADHD symptoms but typically do not adequately address cognitive aspects of ADHD, underscoring the need for new therapeutics. Dopamine and norepinephrine are hypothesized to be particularly important in ADHD, but there is emerging evidence that cholinergic neurotransmission, particularly involving neuronal nicotinic acetylcholine receptors (nAChRs), may play a role in the pathophysiology of ADHD. Nicotine has demonstrated procognitive effects in both humans and experimental animals and has produced signals of efficacy in small proof-of-concept adult ADHD trials. Although adverse effects associated with nicotine preclude its development as a therapeutic, a number of novel nAChR agonists with improved safety/tolerability profiles have been discovered. Of these, ABT-418 and ABT-089 have both demonstrated signals of efficacy in adults with ADHD. Notably, tolerability issues that might be expected of a nAChR agonist, such as nausea and emesis, were not observed at efficacious doses of ABT-089. Further understanding of the effects of novel neuronal nAChR agonists on specific aspects of cognitive functioning in ADHD is required to assess the full potential of this approach.
Conflict of interest statement
Dr. Timothy Wilens receives/d research support from, is/has been a speaker for, or is/has been on the advisory board for the following Pharmaceutical Companies: Abbott Laboratories, Ortho-McNeil, Eli Lilly and Company, National Institute on Drug Abuse (NIDA), Novartis Pharmaceuticals, and Shire Laboratories Inc. Dr. Michael Decker is an employee of Abbott Laboratories. Some of the compounds discussed in detail in the article are Abbott Compounds (ABT-418 & ABT-089).
Figures
References
-
- Goldman L, Genel M, Bezman RJ, Slanetz PJ. Diagnosis and treatment of attention-deficit/hyperactivity disorder in children and adolescents. JAMA. 1998;279:1100–1107. - PubMed
-
- Barkley RA, Fischer M, Edelbrock CS, Smallish L. The adolescent outcome of hyperactive children diagnosed by research criteria: I. An 8-year prospective followup study. J Am Acad Child Adolesc Psychiatry. 1990;29:546–557. - PubMed
-
- Weiss G, Hechtman L, Milroy T, Perlman T. Psychiatric status of hyperactives as adults: A controlled prospective 15 year follow up of 63 hyperactive children. J Am Acad Child Adolesc Psychiatry. 1985;24:211–220. - PubMed
-
- Weiss G. Child and Adolescent Psychiatric Clinics of North America. W.B. Saunders Company; Philadelphia PA: 1992. Attention-Deficit Disorder.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

