Norepinephrine suppresses wound macrophage phagocytic efficiency through alpha- and beta-adrenoreceptor dependent pathways
- PMID: 17689682
- PMCID: PMC2430526
- DOI: 10.1016/j.surg.2007.04.015
Norepinephrine suppresses wound macrophage phagocytic efficiency through alpha- and beta-adrenoreceptor dependent pathways
Abstract
Background: The systemic response to injury is characterized by massive release of norepinephrine (NE) into the circulation as a result of global sympathetic activation. We have recently demonstrated that NE modulates the recruitment of macrophages to the cutaneous wound. We hypothesized that NE suppresses wound macrophage phagocytic function through canonical adrenergic signaling pathways.
Methods: Murine wound macrophages were harvested at 5 days after injury and treated with physiologic and pharmacologic dose norepinephrine. Phagocytosis of green fluorescent protein-labeled Escherichia coli was assayed by flow cytometry. The signaling pathways mediating NE modulation of wound macrophage phagocytosis were interrogated by pharmacologic manipulation of alpha- and beta-adrenoreceptors (ARs), intracellular cyclic adenosine monophosphate (cAMP), and protein kinase A (PKA). Tissue specificity was determined by comparison of wound macrophages to splenic macrophages.
Results: Both physiologic and pharmacologic dose NE suppressed wound macrophage phagocytic efficiency. This effect was mediated by alpha- and beta-ARs in a dose-dependent fashion. Direct stimulation of cAMP-suppressed phagocytic efficiency and blockade of PKA signaling prevented NE-mediated suppression of phagocytic efficiency. Splenic macrophage phagocytic efficiency was less than that of wound macrophages and was not altered by NE.
Conclusions: NE has a profound immunosuppressive effect on wound macrophage function that is tissue specific and appears to be mediated through adrenergic receptors and their canonical downstream signaling pathway. Attenuation of post-injury immunosuppression represents another potential mechanism by which beta-AR blockade may reduce morbidity and mortality after severe injury.
Figures
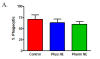




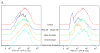

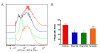


Similar articles
-
Norepinephrine-mediated suppression of phagocytosis by wound neutrophils.J Surg Res. 2009 Apr;152(2):311-8. doi: 10.1016/j.jss.2008.05.001. Epub 2008 Jun 2. J Surg Res. 2009. PMID: 18952237 Free PMC article.
-
cAMP-mediated beta-adrenergic signaling negatively regulates Gq-coupled receptor-mediated fetal gene response in cardiomyocytes.J Mol Cell Cardiol. 2008 Dec;45(6):761-9. doi: 10.1016/j.yjmcc.2008.09.120. Epub 2008 Sep 25. J Mol Cell Cardiol. 2008. PMID: 18851973
-
Norepinephrine induces slow calcium signalling in murine brown preadipocytes through the beta-adrenoceptor/cAMP/protein kinase A pathway.Cell Signal. 2003 Feb;15(2):209-16. doi: 10.1016/s0898-6568(02)00060-8. Cell Signal. 2003. PMID: 12464392
-
Adrenergic regulation of cardiac myocyte apoptosis.J Cell Physiol. 2001 Dec;189(3):257-65. doi: 10.1002/jcp.10024. J Cell Physiol. 2001. PMID: 11748583 Review.
-
Molecular mechanisms underlying β-adrenergic receptor-mediated cross-talk between sympathetic neurons and immune cells.Int J Mol Sci. 2015 Mar 11;16(3):5635-65. doi: 10.3390/ijms16035635. Int J Mol Sci. 2015. PMID: 25768345 Free PMC article. Review.
Cited by
-
Short-term disruption of TGF-β signaling in adult mice renders the aorta vulnerable to hypertension-induced dissection.JCI Insight. 2025 Feb 11;10(6):e182629. doi: 10.1172/jci.insight.182629. JCI Insight. 2025. PMID: 39932797 Free PMC article.
-
Dose-response relationship between norepinephrine and erythropoiesis: evidence for a critical threshold.J Surg Res. 2010 Oct;163(2):e85-90. doi: 10.1016/j.jss.2010.03.051. Epub 2010 Apr 18. J Surg Res. 2010. PMID: 20605580 Free PMC article.
-
ß-adrenergic stimulation increases macrophage CD14 expression and E. coli phagocytosis through PKA signaling mechanisms.J Leukoc Biol. 2010 Oct;88(4):715-24. doi: 10.1189/jlb.0410186. Epub 2010 Jul 19. J Leukoc Biol. 2010. PMID: 20643814 Free PMC article.
-
An evaluation of the effects of nonselective and cardioselective β-blockers on wound healing in Sprague Dawley rats.Indian J Pharmacol. 2012 Sep-Oct;44(5):629-33. doi: 10.4103/0253-7613.100399. Indian J Pharmacol. 2012. PMID: 23112427 Free PMC article.
-
Use of inotropes and vasopressor agents in critically ill patients.Br J Pharmacol. 2012 Apr;165(7):2015-33. doi: 10.1111/j.1476-5381.2011.01588.x. Br J Pharmacol. 2012. PMID: 21740415 Free PMC article. Review.
References
-
- Centers for Disease Control and Prevention: National Center for Injury Prevention and Control. Web-based Injury Statistics Query and Reporting System (WISQARS) www.cdc.gov/ncipc/wisqars. 2005. [cited 2006 May 4]; Available from:
-
- Baker CC, Oppenheimer L, Stephens B, Lewis FR, Trunkey DD. Epidemiology of trauma deaths. Am J Surg. 1980;140(1):144–50. - PubMed
-
- Deitch EA. Infection in the compromised host. Surg Clin North Am. 1988;68(1):181–97. - PubMed
-
- Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev. 2000;52(4):595–638. - PubMed
-
- Woolf PD, McDonald JV, Feliciano DV, Kelly MM, Nichols D, Cox C. The catecholamine response to multisystem trauma. Arch Surg. 1992;127(8):899–903. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

