Phosphatidylinositol 3-kinase pathway regulates hypoxia-inducible factor-1 to protect from intestinal injury during necrotizing enterocolitis
- PMID: 17689699
- PMCID: PMC2613757
- DOI: 10.1016/j.surg.2007.04.018
Phosphatidylinositol 3-kinase pathway regulates hypoxia-inducible factor-1 to protect from intestinal injury during necrotizing enterocolitis
Abstract
Background: Hypoxia is an important risk factor for development of necrotizing enterocolitis (NEC) in premature infants. Hypoxia-inducible factor (HIF)-1 is a transcription factor that plays a critical role in cellular responses to hypoxia and can be induced by phosphatidylinositol 3-kinase (PI3-K) pathway. Activation of the PI3-K and regulation of HIF-1 during NEC have not been elucidated.
Methods: NEC was induced in 3-day-old neonatal mice using hypoxia and artificial formula feedings. Mice were divided into 3 treatment groups: (1) NEC alone, (2) NEC with insulin-like growth factor (IGF)-I, or (3) NEC with Akt1 siRNA treatment. Animals were sacrificed, and intestinal sections were harvested for protein analysis, H&E, and immunohistochemical staining.
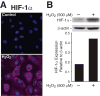
Results: In vivo model of NEC produced intestinal injury associated with increased protein expression of HIF-1alpha, pAkt, PARP, and caspase-3 cleavage. Pretreatment with IGF-1 attenuated an HIF-1alpha response. In contrast, targeted inhibition of Akt1 completely abolished NEC-induced expression of pAkt and upregulated HIF-1alpha activation.
Conclusions: NEC activates important protective cellular responses to hypoxic injury such as HIF-1alpha and PI3-K/Akt in neonatal gut. Hypoxia-mediated activation of pro-survival signaling during NEC may be modulated with growth factors, which thus suggests a potential therapeutic option in the treatment of neonates with NEC.
Figures



References
-
- Sibbons P, Spitz L, van Velzen D, Bullock GR. Relationship of birth weight to the pathogenesis of necrotizing enterocolitis in the neonatal piglet. Pediatr Pathol. 1988;8:151–62. - PubMed
-
- Schumacker PT. Hypoxia-inducible factor-1 (HIF-1) Crit Care Med. 2005;33:S423–5. - PubMed
-
- Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, et al. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

