The tight junction protein, MUPP1, is up-regulated by hypertonicity and is important in the osmotic stress response in kidney cells
- PMID: 17690246
- PMCID: PMC1959440
- DOI: 10.1073/pnas.0702752104
The tight junction protein, MUPP1, is up-regulated by hypertonicity and is important in the osmotic stress response in kidney cells
Abstract
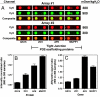
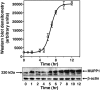
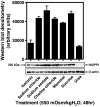
Antibody array proteomics was used to detect differentially expressed proteins in inner medullary collecting duct 3 (IMCD3) cells grown under isotonic and chronic hypertonic conditions. Of 512 potential proteins, >90% were unchanged in expression. Noteworthy was the up-regulation of several tight junction-related proteins, including MUPP1 (multi-PDZ protein-1), ZO1 (zonula occludens 1), and Af6. The most robustly up-regulated protein under hypertonic conditions was MUPP1 (7.2x, P < 0.001). Changes in expression for MUPP1 were verified by quantitative PCR for message and Western blot for protein. In mouse kidney tissues, MUPP1 expression was substantial in the papilla and was absent in the cortex. Furthermore, MUPP1 expression increased 253% (P < 0.01) in the papilla upon 36 h of thirsting. Localization of MUPP1 protein expression was confirmed by immunocytochemical analysis demonstrating only minor staining under isotonic conditions and the substantial presence in chronically adapted cells at the basolateral membrane. Message and protein half-life in IMCD3 cells were 26.2 and 17.8 h, respectively. Osmotic initiators of MUPP1 expression included NaCl, sucrose, mannitol, sodium acetate, and choline chloride but not urea. Stable IMCD3 clones silenced for MUPP1 expression used the pSM2-MUPP1 vector. In cell viability experiments, clones silenced for MUPP1 demonstrated only a minor loss in cell survival under acute sublethal osmotic stress compared with empty vector control cells. In contrast, a 24% loss (P < 0.02) in transepithelial resistance for monolayers of MUPP1-silenced cells was determined as compared with controls. These results suggest that MUPP1 specifically, and potentially tight junction complexes in general, are important in the renal osmoadaptive response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
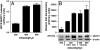




Similar articles
-
Hypertonic stress increases claudin-4 expression and tight junction integrity in association with MUPP1 in IMCD3 cells.Proc Natl Acad Sci U S A. 2008 Oct 14;105(41):15797-802. doi: 10.1073/pnas.0805761105. Epub 2008 Oct 7. Proc Natl Acad Sci U S A. 2008. PMID: 18840681 Free PMC article.
-
Expression of the calcium-binding protein S100A4 is markedly up-regulated by osmotic stress and is involved in the renal osmoadaptive response.J Biol Chem. 2007 Mar 2;282(9):6644-52. doi: 10.1074/jbc.M609432200. Epub 2007 Jan 2. J Biol Chem. 2007. PMID: 17200116 Free PMC article.
-
Silencing and overexpression of the gamma-subunit of Na-K-ATPase directly affect survival of IMCD3 cells in response to hypertonic stress.Am J Physiol Renal Physiol. 2006 Dec;291(6):F1142-7. doi: 10.1152/ajprenal.00077.2006. Epub 2006 Jun 27. Am J Physiol Renal Physiol. 2006. PMID: 16804105
-
Heat shock proteins and the cellular response to osmotic stress.Cell Physiol Biochem. 2000;10(5-6):303-6. doi: 10.1159/000016362. Cell Physiol Biochem. 2000. PMID: 11125209 Review.
-
How do kidney cells adapt to survive in hypertonic inner medulla?Trans Am Clin Climatol Assoc. 2009;120:389-401. Trans Am Clin Climatol Assoc. 2009. PMID: 19768191 Free PMC article. Review.
Cited by
-
Dragon enhances BMP signaling and increases transepithelial resistance in kidney epithelial cells.J Am Soc Nephrol. 2010 Apr;21(4):666-77. doi: 10.1681/ASN.2009050511. Epub 2010 Feb 18. J Am Soc Nephrol. 2010. PMID: 20167703 Free PMC article.
-
Cell models for studying renal physiology.Pflugers Arch. 2008 Oct;457(1):1-15. doi: 10.1007/s00424-008-0507-4. Epub 2008 Apr 22. Pflugers Arch. 2008. PMID: 18427833 Review.
-
The hypertonic environment differentially regulates wild-type CFTR and TNR-CFTR chloride channels.Cell Physiol Biochem. 2010;26(4-5):577-86. doi: 10.1159/000322325. Epub 2010 Oct 29. Cell Physiol Biochem. 2010. PMID: 21063095 Free PMC article.
-
Integrating spatial transcriptomics with single-cell transcriptomics reveals a spatiotemporal gene landscape of the human developing kidney.Cell Biosci. 2022 Jun 3;12(1):80. doi: 10.1186/s13578-022-00801-x. Cell Biosci. 2022. PMID: 35659756 Free PMC article.
-
Sucrose induces fatty liver and pancreatic inflammation in male breeder rats independent of excess energy intake.Metabolism. 2011 Sep;60(9):1259-70. doi: 10.1016/j.metabol.2011.01.008. Epub 2011 Apr 12. Metabolism. 2011. PMID: 21489572 Free PMC article.
References
-
- Burg MB, Kwon ED, Kultz D. Annu Rev Physiol. 1997;59:437–455. - PubMed
-
- Ferraris JD, Burg MB. Contri Nephrol. 2006;152:125–141. - PubMed
-
- Handler JS, Kwon HM. Kidney Int. 1996;49:1682–1683. - PubMed
-
- Handler JS, Kwon HM. Nephron. 2001;87:106–110. - PubMed
-
- Tian W, Cohen DM. Am J Physiol. 2002;283:F388–F398. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

