Nuclear targeting of the growth hormone receptor results in dysregulation of cell proliferation and tumorigenesis
- PMID: 17690250
- PMCID: PMC1948913
- DOI: 10.1073/pnas.0600181104
Nuclear targeting of the growth hormone receptor results in dysregulation of cell proliferation and tumorigenesis
Abstract
Growth hormone receptor (GHR) has been demonstrated to be nuclear localized both in vivo and in vitro, but the significance of this observation has remained elusive. Here we show that nuclear GHR is strongly correlated with proliferative status in vivo by using a liver regeneration model. In vitro, nuclear translocation of the GH receptor is GH-dependent and appears to be mediated by the Importin system. Constitutive nuclear targeting of GHR in murine pro-B cells is associated with constitutive activation of STAT5, a transforming agent in lymphoma and other cell types. This activation is abrogated by inhibition of JAK2 and appears to be driven by autocrine murine GH action coupled with enhanced nuclear uptake of phospho-STAT5. Nuclear targeting induces dysregulated cell cycle progression in the pro-B cell line, associated with constitutive up-regulation of the proliferation inducers Survivin and Mybbp, the metastasis related Dysadherin, and other tumor markers. GHR nuclear-targeted cells generate aggressive metastatic tumors when injected into nude mice, which display nuclear localized GHR strikingly similar to that seen in human lymphomas. We conclude that aberrant nuclear localization of GHR is a marker of high proliferative status and is sufficient to induce tumorigenesis and tumor progression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
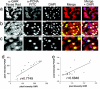

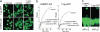
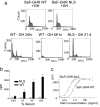


References
-
- Lobie PE, Barnard R, Waters MJ. J Biol Chem. 1991;266:22645–22652. - PubMed
-
- Mertani HC, Raccurt M, Abbate A, Kindblom J, Tornell J, Billestrup N, Usson Y, Morel G, Lobie PE. Endocrinology. 2003;144:3182–3195. - PubMed
-
- Lobie PE, Wood TJ, Chen CM, Waters MJ, Norstedt G. J Biol Chem. 1994;269:31735–31746. - PubMed
-
- Lin SY, Makino K, Xia W, Matin A, Wen Y, Kwong KY, Bourguignon L, Hung MC. Nat Cell Biol. 2001;3:802–808. - PubMed
-
- Johnson HM, Subramaniam PS, Olsnes S, Jans DA. BioEssays. 2004;26:993–1004. - PubMed
Publication types
MeSH terms
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

