Kupffer cell heterogeneity: functional properties of bone marrow derived and sessile hepatic macrophages
- PMID: 17690256
- PMCID: PMC2190614
- DOI: 10.1182/blood-2007-02-073841
Kupffer cell heterogeneity: functional properties of bone marrow derived and sessile hepatic macrophages
Abstract
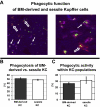
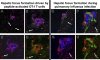
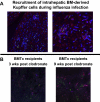
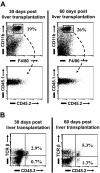
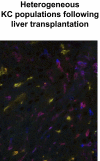
Kupffer cells form a large intravascular macrophage bed in the liver sinusoids. The differentiation history and diversity of Kupffer cells is disputed; some studies argue that they are derived from blood monocytes, whereas others support a local origin from intrahepatic precursor cells. In the present study, we used both flow cytometry and immunohistochemistry to distinguish 2 subsets of Kupffer cells that were revealed in the context both of bone marrow transplantation and of orthotopic liver transplantation. One subset was radiosensitive and rapidly replaced from hematogenous precursors, whereas the other was relatively radioresistant and long-lived. Both were phagocytic but only the former population was recruited into inflammatory foci in response to CD8(+) T-cell activation. We propose the name "sessile" for the radioresistant Kupffer cells that do not participate in immunoinflammatory reactions. However, we found no evidence that these sessile Kupffer cells arise from immature intrahepatic precursors. Our conclusions resolve a long-standing controversy and explain how different experimental approaches may reveal one or both of these subsets.
Figures


References
-
- Bayon LG, Izquierdo MA, Sirovich I, van Rooijen N, Beelen RH, Meijer S. Role of Kupffer cells in arresting circulating tumor cells and controlling metastatic growth in the liver. Hepatology. 1996;23:1224–1231. - PubMed
-
- Lehner PJ, Cresswell P. Recent developments in MHC-class-I-mediated antigen presentation. Curr Opin Immunol. 2004;16:82–89. - PubMed
-
- Rogoff TM, Lipsky PE. Antigen presentation by isolated guinea pig Kupffer cells. J Immunol. 1980;124:1740–1744. - PubMed
-
- Seki S, Habu Y, Kawamura T, et al. The liver as a crucial organ in the first line of host defense: the roles of Kupffer cells, natural killer (NK) cells and NK1. 1 Ag+ T cells in T helper 1 immune responses. Immunol Rev. 2000;174:35–46. - PubMed
-
- Wiegard C, Frenzel C, Herkel J, Kallen KJ, Schmitt E, Lohse AW. Murine liver antigen presenting cells control suppressor activity of CD4+CD25+ regulatory T cells. Hepatology. 2005;42:193–199. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

