Heparan sulfate is a binding molecule but not a receptor for CEACAM1-independent infection of murine coronavirus
- PMID: 17692355
- PMCID: PMC7103320
- DOI: 10.1016/j.virol.2007.06.034
Heparan sulfate is a binding molecule but not a receptor for CEACAM1-independent infection of murine coronavirus
Abstract
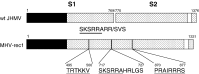
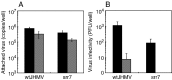
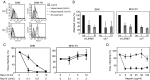
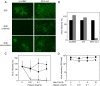
A highly neurovirulent mouse hepatitis virus (MHV) JHMV strain (wt) with receptor (MHVR)-independent infection activity and its low-virulent mutant srr7 without such activity were found to attach to MHVR-negative, non-permissive BHK cells. To identify the molecule that interacts with JHMV, we focused on heparan sulfate (HS) since it works as a receptor of a mutant MHV-rec1 that infects in an MHVR-independent fashion. The present study indicates that HS interacts with both wt JHMV and srr7 but it does not function as an entry receptor as it apparently does for MHV-rec1. Furthermore, HS failed to serve as an entry receptor in the MHVR-independent infection of wt JHMV, indicating that HS is not a host factor that wt JHMV utilizes in an MHVR-independent infection.
Figures
Similar articles
-
Receptor-independent infection of murine coronavirus: analysis by spinoculation.J Virol. 2006 May;80(10):4901-8. doi: 10.1128/JVI.80.10.4901-4908.2006. J Virol. 2006. PMID: 16641281 Free PMC article.
-
Soluble receptor potentiates receptor-independent infection by murine coronavirus.J Virol. 2002 Feb;76(3):950-8. doi: 10.1128/jvi.76.3.950-958.2002. J Virol. 2002. PMID: 11773370 Free PMC article.
-
Receptor-independent spread of a highly neurotropic murine coronavirus JHMV strain from initially infected microglial cells in mixed neural cultures.J Virol. 2005 May;79(10):6102-10. doi: 10.1128/JVI.79.10.6102-6110.2005. J Virol. 2005. PMID: 15857995 Free PMC article.
-
MHVR-independent cell-cell spread of mouse hepatitis virus infection requires neutral pH fusion.Adv Exp Med Biol. 1995;380:351-7. doi: 10.1007/978-1-4615-1899-0_57. Adv Exp Med Biol. 1995. PMID: 8830507 Review.
-
Characterization of a new gene that encodes a functional MHV receptor and progress in the identification of the virus-binding site(s).Adv Exp Med Biol. 1995;380:345-50. doi: 10.1007/978-1-4615-1899-0_56. Adv Exp Med Biol. 1995. PMID: 8830505 Review.
Cited by
-
Anti-SARS-CoV-2 Natural Products as Potentially Therapeutic Agents.Front Pharmacol. 2021 May 27;12:590509. doi: 10.3389/fphar.2021.590509. eCollection 2021. Front Pharmacol. 2021. PMID: 34122058 Free PMC article. Review.
-
Comprehensive Risk Assessment of Infection Induced by SARS-CoV-2.Mol Neurobiol. 2024 Dec;61(12):9851-9872. doi: 10.1007/s12035-023-03682-4. Epub 2023 Oct 11. Mol Neurobiol. 2024. PMID: 37817031 Review.
-
Cell Entry of Animal Coronaviruses.Viruses. 2021 Oct 1;13(10):1977. doi: 10.3390/v13101977. Viruses. 2021. PMID: 34696406 Free PMC article. Review.
-
Membrane Protein of Human Coronavirus NL63 Is Responsible for Interaction with the Adhesion Receptor.J Virol. 2019 Sep 12;93(19):e00355-19. doi: 10.1128/JVI.00355-19. Print 2019 Oct 1. J Virol. 2019. PMID: 31315999 Free PMC article.
-
Effective Inhibition of SARS-CoV-2 Entry by Heparin and Enoxaparin Derivatives.J Virol. 2021 Jan 13;95(3):e01987-20. doi: 10.1128/JVI.01987-20. Print 2021 Jan 13. J Virol. 2021. PMID: 33173010 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

