Liquid movement across the surface epithelium of large airways
- PMID: 17692578
- PMCID: PMC2696130
- DOI: 10.1016/j.resp.2007.06.005
Liquid movement across the surface epithelium of large airways
Abstract
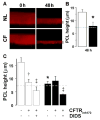
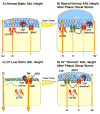
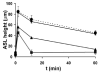
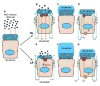
The cystic fibrosis transmembrane conductance regulator CFTR gene is found on chromosome 7 [Kerem, B., Rommens, J.M., Buchanan, J.A., Markiewicz, D., Cox, T.K., Chakravarti, A., Buchwald, M., Tsui, L.C., 1989. Identification of the cystic fibrosis gene: genetic analysis. Science 245, 1073-1080; Riordan, J.R., Rommens, J.M., Kerem, B., Alon, N., Rozmahel, R., Grzelczak, Z., Zielenski, J., Lok, S., Plavsic, N., Chou, J.L., et al., 1989. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 245, 1066-1073] and encodes for a 1480 amino acid protein which is present in the plasma membrane of epithelial cells [Anderson, M.P., Sheppard, D.N., Berger, H.A., Welsh, M.J., 1992. Chloride channels in the apical membrane of normal and cystic fibrosis airway and intestinal epithelia. Am. J. Physiol. 263, L1-L14]. This protein appears to have many functions, but a unifying theme is that it acts as a protein kinase C- and cyclic AMP-regulated Cl(-) channel [Winpenny, J.P., McAlroy, H.L., Gray, M.A., Argent, B.E., 1995. Protein kinase C regulates the magnitude and stability of CFTR currents in pancreatic duct cells. Am. J. Physiol. 268, C823-C828; Jia, Y., Mathews, C.J., Hanrahan, J.W., 1997. Phosphorylation by protein kinase C is required for acute activation of cystic fibrosis transmembrane conductance regulator by protein kinase A. J. Biol. Chem. 272, 4978-4984]. In the superficial epithelium of the conducting airways, CFTR is involved in Cl(-) secretion [Boucher, R.C., 2003. Regulation of airway surface liquid volume by human airway epithelia. Pflugers Arch. 445, 495-498] and also acts as a regulator of the epithelial Na(+) channel (ENaC) and hence Na(+) absorption [Boucher, R.C., Stutts, M.J., Knowles, M.R., Cantley, L., Gatzy, J.T., 1986. Na(+) transport in cystic fibrosis respiratory epithelia. Abnormal basal rate and response to adenylate cyclase activation. J. Clin. Invest. 78, 1245-1252; Stutts, M.J., Canessa, C.M., Olsen, J.C., Hamrick, M., Cohn, J.A., Rossier, B.C., Boucher, R.C., 1995. CFTR as a cAMP-dependent regulator of sodium channels. Science 269, 847-850]. In this chapter, we will discuss the regulation of these two ion channels, and how they can influence liquid movement across the superficial airway epithelium.
Figures
Similar articles
-
Expression and function of Anoctamin 1/TMEM16A calcium-activated chloride channels in airways of in vivo mouse models for cystic fibrosis research.Pflugers Arch. 2018 Sep;470(9):1335-1348. doi: 10.1007/s00424-018-2160-x. Epub 2018 Jun 2. Pflugers Arch. 2018. PMID: 29860639
-
Human cystic fibrosis airway epithelia have reduced Cl- conductance but not increased Na+ conductance.Proc Natl Acad Sci U S A. 2011 Jun 21;108(25):10260-5. doi: 10.1073/pnas.1106695108. Epub 2011 Jun 6. Proc Natl Acad Sci U S A. 2011. PMID: 21646513 Free PMC article.
-
CFTR delivery to 25% of surface epithelial cells restores normal rates of mucus transport to human cystic fibrosis airway epithelium.PLoS Biol. 2009 Jul;7(7):e1000155. doi: 10.1371/journal.pbio.1000155. Epub 2009 Jul 21. PLoS Biol. 2009. PMID: 19621064 Free PMC article.
-
Local regulation of cystic fibrosis transmembrane regulator and epithelial sodium channel in airway epithelium.Proc Am Thorac Soc. 2004;1(1):33-7. doi: 10.1513/pats.2306012. Proc Am Thorac Soc. 2004. PMID: 16113409 Review.
-
[CFTR and ENaC functions in cystic fibrosis].Medicina (B Aires). 2014;74(2):133-9. Medicina (B Aires). 2014. PMID: 24736260 Review. Spanish.
Cited by
-
Does epithelial sodium channel hyperactivity contribute to cystic fibrosis lung disease?J Physiol. 2013 Sep 15;591(18):4377-87. doi: 10.1113/jphysiol.2012.240861. Epub 2013 Jul 22. J Physiol. 2013. PMID: 23878362 Free PMC article. Review.
-
Exhaled breath condensate detects baseline reductions in chloride and increases in response to albuterol in cystic fibrosis patients.Clin Med Insights Circ Respir Pulm Med. 2013 Dec 10;7:79-90. doi: 10.4137/CCRPM.S12882. eCollection 2013. Clin Med Insights Circ Respir Pulm Med. 2013. PMID: 24367235 Free PMC article.
-
Suppression of CFTR-mediated Cl secretion of airway epithelium in vitamin C-deficient mice.J Korean Med Sci. 2011 Mar;26(3):317-24. doi: 10.3346/jkms.2011.26.3.317. Epub 2011 Feb 25. J Korean Med Sci. 2011. PMID: 21394297 Free PMC article.
-
The Effects of Individual Components of E-Cigarettes on Ion Transport and Airway Surface Liquid Height in Human Bronchial Epithelial Cells.Medicina (Kaunas). 2025 Mar 17;61(3):526. doi: 10.3390/medicina61030526. Medicina (Kaunas). 2025. PMID: 40142337 Free PMC article.
-
Silencing human genetic diseases with oligonucleotide-based therapies.Hum Genet. 2013 May;132(5):481-93. doi: 10.1007/s00439-013-1288-1. Epub 2013 Mar 14. Hum Genet. 2013. PMID: 23494242 Review.
References
-
- Anderson MP, Sheppard DN, Berger HA, Welsh MJ. Chloride channels in the apical membrane of normal and cystic fibrosis airway and intestinal epithelia. Am J Physiol. 1992;263:L1–14. - PubMed
-
- Bachhuber T, Konig J, Voelcker T, Murle B, Schreiber R, Kunzelmann K. Cl-interference with the epithelial Na+ channel ENaC. J Biol Chem. 2005;280:31587–31594. - PubMed
-
- Ballard ST, Trout L, Mehta A, Inglis SK. Liquid secretion inhibitors reduce mucociliary transport in glandular airways. Am J Physiol Lung Cell Mol Physiol. 2002;283:L329–335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

