Human alpha B-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice
- PMID: 17693254
- PMCID: PMC2962423
- DOI: 10.1016/j.cell.2007.06.044
Human alpha B-crystallin mutation causes oxido-reductive stress and protein aggregation cardiomyopathy in mice
Abstract
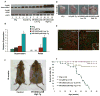
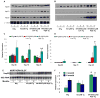
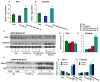
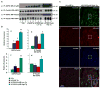
The autosomal dominant mutation in the human alphaB-crystallin gene inducing a R120G amino acid exchange causes a multisystem, protein aggregation disease including cardiomyopathy. The pathogenesis of cardiomyopathy in this mutant (hR120GCryAB) is poorly understood. Here, we show that transgenic mice overexpressing cardiac-specific hR120GCryAB recapitulate the cardiomyopathy in humans and find that the mice are under reductive stress. The myopathic hearts show an increased recycling of oxidized glutathione (GSSG) to reduced glutathione (GSH), which is due to the augmented expression and enzymatic activities of glucose-6-phosphate dehydrogenase (G6PD), glutathione reductase, and glutathione peroxidase. The intercross of hR120GCryAB cardiomyopathic animals with mice with reduced G6PD levels rescues the progeny from cardiac hypertrophy and protein aggregation. These findings demonstrate that dysregulation of G6PD activity is necessary and sufficient for maladaptive reductive stress and suggest a novel therapeutic target for abrogating R120GCryAB cardiomyopathy and heart failure in humans.
Figures


Comment in
-
A "reductionist" view of cardiomyopathy.Cell. 2007 Aug 10;130(3):401-2. doi: 10.1016/j.cell.2007.07.028. Cell. 2007. PMID: 17693248 Review.
References
-
- Baek SH, Min JN, Park EM, Han MY, Lee YS, Lee YJ, Park YM. Role of small heat shock protein HSP25 in radioresistance and glutathione-redox cycle. J Cell Physiol. 2000;183:100–107. - PubMed
-
- Bukau B, Weissman J, Horwich A. Molecular chaperones and protein quality control. Cell. 2006;125:443–451. - PubMed
-
- Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

