Modeling hypertrophic IP3 transients in the cardiac myocyte
- PMID: 17693463
- PMCID: PMC2072074
- DOI: 10.1529/biophysj.107.110031
Modeling hypertrophic IP3 transients in the cardiac myocyte
Abstract
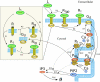
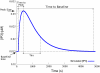
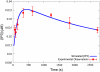
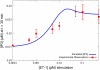
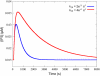
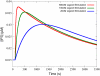
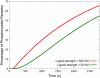
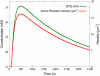
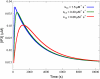
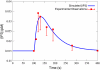
Cardiac hypertrophy is a known risk factor for heart disease, and at the cellular level is caused by a complex interaction of signal transduction pathways. The IP3-calcineurin pathway plays an important role in stimulating the transcription factor NFAT which binds to DNA cooperatively with other hypertrophic transcription factors. Using available kinetic data, we construct a mathematical model of the IP3 signal production system after stimulation by a hypertrophic alpha-adrenergic agonist (endothelin-1) in the mouse atrial cardiac myocyte. We use a global sensitivity analysis to identify key controlling parameters with respect to the resultant IP3 transient, including the phosphorylation of cell-membrane receptors, the ligand strength and binding kinetics to precoupled (with G(alpha)GDP) receptor, and the kinetics associated with precoupling the receptors. We show that the kinetics associated with the receptor system contribute to the behavior of the system to a great extent, with precoupled receptors driving the response to extracellular ligand. Finally, by reparameterizing for a second hypertrophic alpha-adrenergic agonist, angiotensin-II, we show that differences in key receptor kinetic and membrane density parameters are sufficient to explain different observed IP3 transients in essentially the same pathway.
Figures
Similar articles
-
Nuclear inositol 1,4,5-trisphosphate is a necessary and conserved signal for the induction of both pathological and physiological cardiomyocyte hypertrophy.J Mol Cell Cardiol. 2012 Oct;53(4):475-86. doi: 10.1016/j.yjmcc.2012.06.017. Epub 2012 Jul 2. J Mol Cell Cardiol. 2012. PMID: 22766271
-
Human embryonic stem cell-derived cardiomyocytes can mobilize 1,4,5-inositol trisphosphate-operated [Ca2+]i stores: the functionality of angiotensin-II/endothelin-1 signaling pathways.Ann N Y Acad Sci. 2010 Feb;1188:68-77. doi: 10.1111/j.1749-6632.2009.05085.x. Ann N Y Acad Sci. 2010. PMID: 20201888
-
The 1,4,5-inositol trisphosphate pathway is a key component in Fas-mediated hypertrophy in neonatal rat ventricular myocytes.Cardiovasc Res. 2005 Oct 1;68(1):75-86. doi: 10.1016/j.cardiores.2005.05.015. Cardiovasc Res. 2005. PMID: 16005448
-
Direct and indirect interactions between calcineurin-NFAT and MEK1-extracellular signal-regulated kinase 1/2 signaling pathways regulate cardiac gene expression and cellular growth.Mol Cell Biol. 2005 Feb;25(3):865-78. doi: 10.1128/MCB.25.3.865-878.2005. Mol Cell Biol. 2005. PMID: 15657416 Free PMC article.
-
Signaling pathways activated by vasoactive peptides in the cardiac myocyte and their role in myocardial pathologies.J Card Fail. 2002 Dec;8(6 Suppl):S359-69. doi: 10.1054/jcaf.2002.129282. J Card Fail. 2002. PMID: 12555146 Review.
Cited by
-
CellML metadata standards, associated tools and repositories.Philos Trans A Math Phys Eng Sci. 2009 May 28;367(1895):1845-67. doi: 10.1098/rsta.2008.0310. Philos Trans A Math Phys Eng Sci. 2009. PMID: 19380315 Free PMC article.
-
Mechanical regulation of gene expression in cardiac myocytes and fibroblasts.Nat Rev Cardiol. 2019 Jun;16(6):361-378. doi: 10.1038/s41569-019-0155-8. Nat Rev Cardiol. 2019. PMID: 30683889 Free PMC article. Review.
-
Modular modelling with Physiome standards.J Physiol. 2016 Dec 1;594(23):6817-6831. doi: 10.1113/JP272633. Epub 2016 Aug 29. J Physiol. 2016. PMID: 27353233 Free PMC article.
-
Mathematical modeling of cardiac growth and remodeling.Wiley Interdiscip Rev Syst Biol Med. 2016 May;8(3):211-26. doi: 10.1002/wsbm.1330. Epub 2016 Mar 7. Wiley Interdiscip Rev Syst Biol Med. 2016. PMID: 26952285 Free PMC article. Review.
-
Emerging roles of inositol 1,4,5-trisphosphate signaling in cardiac myocytes.J Mol Cell Cardiol. 2008 Aug;45(2):128-47. doi: 10.1016/j.yjmcc.2008.05.014. Epub 2008 Jun 15. J Mol Cell Cardiol. 2008. PMID: 18603259 Free PMC article. Review.
References
-
- Heineke, J., and J. D. Molkentin. 2006. Regulation of cardiac hypertrophy by intracellular signaling pathways. Nat. Rev. Mol. Cell Biol. 7:589–600. - PubMed
-
- Sugden, P. H. 1999. Signaling in myocardial hypertrophy: life after calcineurin? Circ. Res. 84:633–646. - PubMed
-
- Bare, D. J., C. S. Kettlun, M. Liang, D. M. Bers, and G. A. Mignery. 2005. Cardiac type 2 inositol 1,4,5-trisphosphate receptor: interaction and modulation by calcium/calmodulin-dependent protein kinase II. J. Biol. Chem. 280:15912–15920. - PubMed
-
- Hardt, S. E., and J. Sadoshima. 2002. Glycogen synthase kinase-3β: a novel regulator of cardiac hypertrophy and development. Circ. Res. 90:1055–1063. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

