A ribonuclease III domain protein functions in group II intron splicing in maize chloroplasts
- PMID: 17693527
- PMCID: PMC2002627
- DOI: 10.1105/tpc.107.053736
A ribonuclease III domain protein functions in group II intron splicing in maize chloroplasts
Abstract
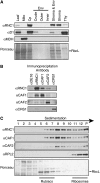
Chloroplast genomes in land plants harbor approximately 20 group II introns. Genetic approaches have identified proteins involved in the splicing of many of these introns, but the proteins identified to date cannot account for the large size of intron ribonucleoprotein complexes and are not sufficient to reconstitute splicing in vitro. Here, we describe an additional protein that promotes chloroplast group II intron splicing in vivo. This protein, RNC1, was identified by mass spectrometry analysis of maize (Zea mays) proteins that coimmunoprecipitate with two previously identified chloroplast splicing factors, CAF1 and CAF2. RNC1 is a plant-specific protein that contains two ribonuclease III (RNase III) domains, the domain that harbors the active site of RNase III and Dicer enzymes. However, several amino acids that are essential for catalysis by RNase III and Dicer are missing from the RNase III domains in RNC1. RNC1 is found in complexes with a subset of chloroplast group II introns that includes but is not limited to CAF1- and CAF2-dependent introns. The splicing of many of the introns with which it associates is disrupted in maize rnc1 insertion mutants, indicating that RNC1 facilitates splicing in vivo. Recombinant RNC1 binds both single-stranded and double-stranded RNA with no discernible sequence specificity and lacks endonuclease activity. These results suggest that RNC1 is recruited to specific introns via protein-protein interactions and that its role in splicing involves RNA binding but not RNA cleavage activity.
Figures



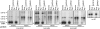
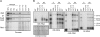

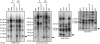


References
-
- Barkan, A. (1998). Approaches to investigating nuclear genes that function in chloroplast biogenesis in land plants. Methods Enzymol. 297 38–57.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

