Medicago truncatula as a model for nonhost resistance in legume-parasitic plant interactions
- PMID: 17693539
- PMCID: PMC2048724
- DOI: 10.1104/pp.107.097089
Medicago truncatula as a model for nonhost resistance in legume-parasitic plant interactions
Abstract
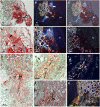
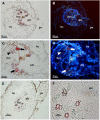
Crenate broomrape (Orobanche crenata) is a root parasitic weed that represents a major constraint for grain legume production in Mediterranean and West Asian countries. Medicago truncatula has emerged as an important model plant species for structural and functional genomics. The close phylogenic relationship of M. truncatula with crop legumes increases its value as a resource for understanding resistance against Orobanche spp. Different cytological methods were used to study the mechanisms of resistance against crenate broomrape of two accessions of M. truncatula, showing early and late acting resistance. In the early resistance accession (SA27774) we found that the parasite died before a tubercle had formed. In the late resistance accession (SA4327) the parasite became attached without apparent problems to the host roots but most of the established tubercles turned dark and died before emergence. The results suggest that there are defensive mechanisms acting in both accessions but with a time gap that is crucial for a higher success avoiding parasite infection.
Figures






Similar articles
-
Differential expression proteomics to investigate responses and resistance to Orobanche crenata in Medicago truncatula.BMC Genomics. 2009 Jul 3;10:294. doi: 10.1186/1471-2164-10-294. BMC Genomics. 2009. PMID: 19575787 Free PMC article.
-
Interaction between Orobanche crenata and its host legumes: unsuccessful haustorial penetration and necrosis of the developing parasite.Ann Bot. 2005 May;95(6):935-42. doi: 10.1093/aob/mci105. Epub 2005 Mar 4. Ann Bot. 2005. PMID: 15749751 Free PMC article.
-
Gene expression analysis of molecular mechanisms of defense induced in Medicago truncatula parasitized by Orobanche crenata.Plant Physiol Biochem. 2009 Jul;47(7):635-41. doi: 10.1016/j.plaphy.2009.02.016. Epub 2009 Mar 10. Plant Physiol Biochem. 2009. PMID: 19321356
-
Breeding approaches for crenate broomrape (Orobanche crenata Forsk.) management in pea (Pisum sativum L.).Pest Manag Sci. 2009 May;65(5):553-9. doi: 10.1002/ps.1740. Pest Manag Sci. 2009. PMID: 19253919 Review.
-
Annual Medicago: from a model crop challenged by a spectrum of necrotrophic pathogens to a model plant to explore the nature of disease resistance.Ann Bot. 2006 Dec;98(6):1117-28. doi: 10.1093/aob/mcl132. Epub 2006 Jun 27. Ann Bot. 2006. PMID: 16803846 Free PMC article. Review.
Cited by
-
The changes of GA level and signaling are involved in the regulation of mesocotyl elongation during blue light mediated de-etiolation in Sorghum bicolor.Mol Biol Rep. 2012 Apr;39(4):4091-100. doi: 10.1007/s11033-011-1191-6. Epub 2011 Jul 31. Mol Biol Rep. 2012. PMID: 21805343
-
Apoplastic interactions between plants and plant root intruders.Front Plant Sci. 2015 Aug 14;6:617. doi: 10.3389/fpls.2015.00617. eCollection 2015. Front Plant Sci. 2015. PMID: 26322059 Free PMC article. Review.
-
Heinz-resistant tomato cultivars exhibit a lignin-based resistance to field dodder (Cuscuta campestris) parasitism.Plant Physiol. 2022 May 3;189(1):129-151. doi: 10.1093/plphys/kiac024. Plant Physiol. 2022. PMID: 35099559 Free PMC article.
-
Biological and Transcriptomic Characterization of Pre-Haustorial Resistance to Sunflower Broomrape (Orobanche cumana W.) in Sunflowers (Helianthus annuus).Plants (Basel). 2021 Aug 30;10(9):1810. doi: 10.3390/plants10091810. Plants (Basel). 2021. PMID: 34579343 Free PMC article.
-
Exploring the biochemical dynamics in faba bean (Vicia faba L. minor) in response to Orobanche foetida Poir. parasitism under inoculation with different rhizobia strains.PLoS One. 2024 May 31;19(5):e0304673. doi: 10.1371/journal.pone.0304673. eCollection 2024. PLoS One. 2024. PMID: 38820398 Free PMC article.
References
-
- Antonova TS, Ter Borg SJ (1996) The role of peroxidase in the resistance of sunflower against Orobanche cumana in Russia. Weed Res 36 113–121
-
- Baayen RP, Ouellette GB, Rioux D (1996) Compartmentalization of decay in carnations resistant to Fusarium oxysporum f. sp. dianthi. Phytopathology 86 1018–1031
-
- Blondon F, Marie D, Brown S, Kondorosi A (1994) Genome size and base composition in Medicago sativa and M. truncatula species. Genome 37 264–275 - PubMed
-
- Bordallo JJ, Lopez-Llorca LV, Jansson HB, Salinas J, Persmark L, Asensio L (2002) Colonization of plant roots by egg-parasitic and nematode-trapping fungi. New Phytol 154 491–499 - PubMed
-
- Cachinero JM, Hervás A, Jiménez-Díaz RM, Tena M (2002) Plant defence reactions against fusarium wilt in chickpea induced by incompatible race 0 of Fusarium oxysporum f.sp. ciceris and nonhost isolates of F. oxysporum. Plant Pathol 51 765–776
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

