Estrogen receptor beta is essential for sprouting of nociceptive primary afferents and for morphogenesis and maintenance of the dorsal horn interneurons
- PMID: 17693550
- PMCID: PMC1959444
- DOI: 10.1073/pnas.0705936104
Estrogen receptor beta is essential for sprouting of nociceptive primary afferents and for morphogenesis and maintenance of the dorsal horn interneurons
Abstract
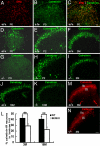
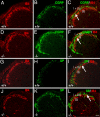
Estrogen is known to influence pain, but the specific roles of the two estrogen receptors (ERs) in the spinal cord are unknown. In the present study, we have examined the expression of ERalpha and ERbeta in the spinal cord and have looked for defects in pain pathways in ERbeta knockout (ERbeta(-/-)) mice. In the spinal cords of 10-month-old WT mice, ERbeta-positive cells were localized in lamina II, whereas ERalpha-positive cells were mainly localized in lamina I. In ERbeta(-/-) mice, there were higher levels of calcitonin gene-regulated peptide and substance P in spinal cord dorsal horn and isolectin B4 in the dorsal root ganglion. In the superficial layers of the spinal cord, there was a decrease in the number of calretinin (CR)-positive neurons, and in the outer layer II, there was a loss of calbindin-positive interneurons. During embryogenesis, ERbeta was first detectable in the spinal cord at embryonic day 13.5 (E13.5), and ERalpha was first detectable at E15.5. During middle and later embryonic stages, ERbeta was abundantly expressed in the superficial layers of the dorsal horn. ERalpha was also expressed in the dorsal horn but was limited to fewer neurons. Double staining for ERbeta and CR showed that, in the superficial dorsal horn of WT neonates [postnatal day 0 (P0)], most CR neurons also expressed ERbeta. At this stage, few CR-positive cells were detected in the dorsal horn of ERbeta(-/-) mice. Taken together, these findings suggest that, early in embryogenesis, ERbeta is involved in dorsal horn morphogenesis and in sensory afferent fiber projections to the dorsal horn and that ERbeta is essential for survival of dorsal horn interneurons throughout life.
Conflict of interest statement
Conflict of interest statement: J.-Å.G. is a shareholder and consultant of Karo Bio AB.
Figures




Similar articles
-
The neuropeptide tyrosine Y1R is expressed in interneurons and projection neurons in the dorsal horn and area X of the rat spinal cord.Neuroscience. 2006;138(4):1361-76. doi: 10.1016/j.neuroscience.2005.11.069. Epub 2006 Jan 31. Neuroscience. 2006. PMID: 16448775
-
Ablation of spinal cord estrogen receptor α-expressing interneurons reduces chemically induced modalities of pain and itch.J Comp Neurol. 2020 Jul;528(10):1629-1643. doi: 10.1002/cne.24847. Epub 2020 Jan 6. J Comp Neurol. 2020. PMID: 31872868 Free PMC article.
-
Differential expression of the estrogen receptors alpha and beta during postnatal development of the rat cerebellum.Brain Res. 2006 Apr 14;1083(1):39-49. doi: 10.1016/j.brainres.2006.02.025. Epub 2006 Mar 20. Brain Res. 2006. PMID: 16542644
-
Specification of dorsal spinal cord interneurons.Curr Opin Neurobiol. 2003 Feb;13(1):42-9. doi: 10.1016/s0959-4388(03)00010-2. Curr Opin Neurobiol. 2003. PMID: 12593981 Review.
-
Role of estrogen receptor alpha and beta expression and signaling on cognitive function during aging.Hippocampus. 2012 Apr;22(4):656-69. doi: 10.1002/hipo.20935. Epub 2011 Apr 27. Hippocampus. 2012. PMID: 21538657 Free PMC article. Review.
Cited by
-
Alterations of gene expression of sodium channels in dorsal root ganglion neurons of estrogen receptor knockout (ERKO) mice induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP).Endocrine. 2012 Aug;42(1):118-24. doi: 10.1007/s12020-012-9637-8. Epub 2012 Feb 28. Endocrine. 2012. PMID: 22371119
-
Expression of estrogen receptor GPR30 in the rat spinal cord and in autonomic and sensory ganglia.J Neurosci Res. 2009 May 15;87(7):1610-9. doi: 10.1002/jnr.21980. J Neurosci Res. 2009. PMID: 19125412 Free PMC article.
-
Estrogen receptor β activation is antinociceptive in a model of visceral pain in the rat.J Pain. 2012 Jul;13(7):685-94. doi: 10.1016/j.jpain.2012.04.010. Epub 2012 Jun 13. J Pain. 2012. PMID: 22698981 Free PMC article.
-
Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders.Mol Psychiatry. 2020 Oct;25(10):2251-2274. doi: 10.1038/s41380-019-0639-2. Epub 2020 Jan 3. Mol Psychiatry. 2020. PMID: 31900428 Review.
-
Radial glia, the keystone of the development of the hippocampal dentate gyrus.Mol Neurobiol. 2015 Feb;51(1):131-41. doi: 10.1007/s12035-014-8692-y. Epub 2014 Apr 10. Mol Neurobiol. 2015. PMID: 24719081 Review.
References
-
- McEwen BS, Alves SE. Endocr Rev. 1999;20:279–307. - PubMed
-
- McEwen BS. J Appl Physiol. 2001;91:2785–2801. - PubMed
-
- Behl C, Manthey D. J Neurocytol. 2000;29:351–358. - PubMed
-
- Beyer C. Anat Embryol. 1999;199:379–390. - PubMed
-
- Chaovipoch P, Jelks KA, Gerhold LM, West EJ, Chongthammakun S, Floyd CL. J Neurotrauma. 2006;23:830–852. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

