Randomized control trial of peer-delivered, modified directly observed therapy for HAART in Mozambique
- PMID: 17693890
- PMCID: PMC4044044
- DOI: 10.1097/QAI.0b013e318153f7ba
Randomized control trial of peer-delivered, modified directly observed therapy for HAART in Mozambique
Abstract
Objective: To assess the efficacy of a peer-delivered intervention to promote short-term (6-month) and long-term (12-month) adherence to HAART in a Mozambican clinic population.
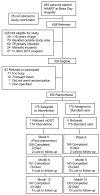
Design: A 2-arm randomized controlled trial was conducted between October 2004 and June 2006.
Participants: Of 350 men and women (> or = 18 years) initiating HAART, 53.7% were female, and 97% were on 1 fixed-dose combination pill twice a day.
Intervention: Participants were randomly assigned to receive 6 weeks (Monday through Friday; 30 daily visits) of peer-delivered, modified directly observed therapy (mDOT) or standard care. Peers provided education about treatment and adherence and sought to identify and mitigate adherence barriers.
Outcome: Participants' self-reported medication adherence was assessed 6 months and 12 months after starting HAART. Adherence was defined as the proportion of prescribed doses taken over the previous 7 days. Statistical analyses were performed using intention-to-treat (missing = failure).
Results: Intervention participants, compared to those in standard care, showed significantly higher mean medication adherence at 6 months (92.7% vs. 84.9%, difference 7.8, 95% confidence interval [CI]: 0.0.02, 13.0) and 12 months (94.4% vs. 87.7%, difference 6.8, 95% CI: 0.9, 12.9). There were no between-arm differences in chart-abstracted CD4 counts.
Conclusions: A peer-delivered mDOT program may be an effective strategy to promote long-term adherence among persons initiating HAART in resource-poor settings.
References
-
- Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. - PubMed
-
- Bangsberg DR, Perry S, Charlebois ED, et al. Non-adherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS. 2001;15:1181–1183. - PubMed
-
- Parruti G, Manzoli L, Toro PM, et al. Long-term adherence to first-line highly active antiretroviral therapy in a hospital-based cohort: predictors and impact on virologic response and relapse. AIDS Patient Care STDS. 2006;20:48–56. - PubMed
-
- Friedland GH, Williams A. Attaining higher goals in HIV treatment: the central importance of adherence. AIDS. 1999;13(Suppl 1):S61–S72. - PubMed
-
- Liu H, Golin CE, Miller LG, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann Intern Med. 2001;134:968–977. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials