Microwave-assisted synthesis of substituted hexahydro-pyrrolo[3,2-c]quinolines
- PMID: 17693952
- PMCID: PMC6149385
- DOI: 10.3390/12010049
Microwave-assisted synthesis of substituted hexahydro-pyrrolo[3,2-c]quinolines
Abstract
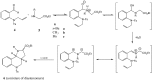
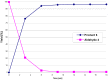
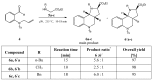
New compounds with the ethyl hexahydro-1H-pyrrolo[3,2-c]quinoline-2-carboxylate skeleton were prepared by microwave-assisted intramolecular 1,3-dipolar cycloaddition reactions. The reactions were carried out under solvent-free conditions and compared with the same reaction in the presence of a solvent and a catalyst. Steric effects on the selectivity of the reaction were noted and evaluated.
Figures
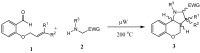
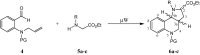




Similar articles
-
Facile synthesis of spiro[indoline-3,3'-pyrrolo[1,2-a]quinolines] and spiro[indoline-3,1'-pyrrolo[2,1-a]isoquinolines] via 1,3-dipolar cycloaddition reactions of heteroaromatic ammonium salts with 3-phenacylideneoxindoles.Org Biomol Chem. 2012 Dec 21;10(47):9452-63. doi: 10.1039/c2ob26849c. Epub 2012 Nov 1. Org Biomol Chem. 2012. PMID: 23117483
-
Combinatorial synthesis of pyrrolo[3,2-f]quinoline and pyrrolo[3,2-a]acridine derivatives via a three-component reaction under catalyst-free conditions.ACS Comb Sci. 2013 Sep 9;15(9):498-502. doi: 10.1021/co400073m. Epub 2013 Aug 16. ACS Comb Sci. 2013. PMID: 23914831
-
Efficient one-pot synthesis of new 1-amino substituted pyrrolo[1,2-a]quinoline-4-carboxylate esters via copper-free Sonogashira coupling reactions.Mol Divers. 2017 Feb;21(1):29-36. doi: 10.1007/s11030-016-9694-7. Epub 2016 Sep 6. Mol Divers. 2017. PMID: 27599493
-
Recent Progress in the Synthesis of Quinolines.Curr Org Synth. 2019;16(5):671-708. doi: 10.2174/1570179416666190719112423. Curr Org Synth. 2019. PMID: 31984888 Review.
-
The Catalysts-Based Synthetic Approaches to Quinolines: A Review.Med Chem. 2024;20(10):921-937. doi: 10.2174/0115734064315729240610045009. Med Chem. 2024. PMID: 38918990 Review.
Cited by
-
Extraction with SPME and synthesis of 2-methyl-6-vinylpyrazine by a 'one pot' reaction using microwaves.Molecules. 2009 Jun 15;14(6):2160-6. doi: 10.3390/molecules14062160. Molecules. 2009. PMID: 19553889 Free PMC article.
-
1,3-Dipolar Cycloaddition in the Preparation of New Fused Heterocyclic Compounds via Thermal Initiation.Molecules. 2016 Feb 4;21(2):187. doi: 10.3390/molecules21020187. Molecules. 2016. PMID: 26861268 Free PMC article.
References
-
- Snider B .B., Ahn Y., Foxman B. M. Synthesis of the tricyclic triamine core of martinelline and martinellic acid. Tetrahedron Lett. 1999;40:3339–3342.
-
- Hadden M., Stevenson P. J. Regioselective synthesis of pyrroloquinolines - Approaches to martinelline. Tetrahedron Lett. 1999;40:1215–1218.
-
- Nyerges M., Fejes I., Töke L. An intermolecular 1,3-dipolar cycloaddition approach to the tricyclic core of martinelline and martinellic acid. Tetrahedron Lett. 2000;41:7951–7954.
-
- Ho T. C. T., Jones K. A synthesis of the tricyclic pyrroloquinoline core of martinelline. Tetrahedron. 1997;53:8287–8294.
-
- Lovely C. J., Mahmud H. An approach to the pyrroloquinoline core of martinelline and martinellic acid. Tetrahedron Lett. 1999;40:2079–2082. doi: 10.1016/S0040-4039(99)00150-1. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

