Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure
- PMID: 17694179
- PMCID: PMC1937500
- DOI: 10.1172/JCI31060
Ca2+- and mitochondrial-dependent cardiomyocyte necrosis as a primary mediator of heart failure
Abstract
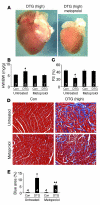
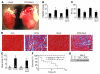
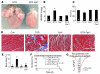
Loss of cardiac myocytes in heart failure is thought to occur largely through an apoptotic process. Here we show that heart failure can also be precipitated through myocyte necrosis associated with Ca2+ overload. Inducible transgenic mice with enhanced sarcolemmal L-type Ca2+ channel (LTCC) activity showed progressive myocyte necrosis that led to pump dysfunction and premature death, effects that were dramatically enhanced by acute stimulation of beta-adrenergic receptors. Enhanced Ca2+ influx-induced cellular necrosis and cardiomyopathy was prevented with either LTCC blockers or beta-adrenergic receptor antagonists, demonstrating a proximal relationship among beta-adrenergic receptor function, Ca2+ handling, and heart failure progression through necrotic cell loss. Mechanistically, loss of cyclophilin D, a regulator of the mitochondrial permeability transition pore that underpins necrosis, blocked Ca2+ influx-induced necrosis of myocytes, heart failure, and isoproterenol-induced premature death. In contrast, overexpression of the antiapoptotic factor Bcl-2 was ineffective in mitigating heart failure and death associated with excess Ca2+ influx and acute beta-adrenergic receptor stimulation. This paradigm of mitochondrial- and necrosis-dependent heart failure was also observed in other mouse models of disease, which supports the concept that heart failure is a pleiotropic disorder that involves not only apoptosis, but also necrotic loss of myocytes in association with dysregulated Ca2+ handling and beta-adrenergic receptor signaling.
Figures

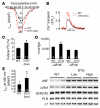

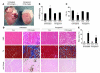

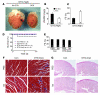
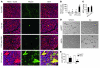

References
-
- Hobbs R.E. Guidelines for the diagnosis and management of heart failure. Am. J. Ther. 2004;11:467–472. - PubMed
-
- Levy D., et al. Long-term trends in the incidence of and survival with heart failure. N. Engl. J. Med. 2002;347:1397–1402. - PubMed
-
- Zannad F., et al. Incidence, clinical and etiologic features, and outcomes of advanced chronic heart failure: the EPICAL Study. Epidemiologie de l’Insuffisance Cardiaque Avancee en Lorraine. . J. Am. Coll. Cardiol. 1999;33:734–742. - PubMed
-
- Haldeman G.A., Croft J.B., Giles W.H., Rashidee A. Hospitalization of patients with heart failure: National Hospital Discharge Survey, 1985 to 1995. Am. Heart. J. 1999;137:352–360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

