Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages
- PMID: 17696608
- PMCID: PMC1941748
- DOI: 10.1371/journal.ppat.0030111
Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages
Abstract
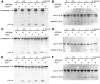
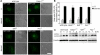
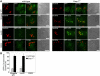
Shigella infection, the cause of bacillary dysentery, induces caspase-1 activation and cell death in macrophages, but the precise mechanisms of this activation remain poorly understood. We demonstrate here that caspase-1 activation and IL-1beta processing induced by Shigella are mediated through Ipaf, a cytosolic pattern-recognition receptor of the nucleotide-binding oligomerization domain (NOD)-like receptor (NLR) family, and the adaptor protein apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain (ASC). We also show that Ipaf was critical for pyroptosis, a specialized form of caspase-1-dependent cell death induced in macrophages by bacterial infection, whereas ASC was dispensable. Unlike that observed in Salmonella and Legionella, caspase-1 activation induced by Shigella infection was independent of flagellin. Notably, infection of macrophages with Shigella induced autophagy, which was dramatically increased by the absence of caspase-1 or Ipaf, but not ASC. Autophagy induced by Shigella required an intact bacterial type III secretion system but not VirG protein, a bacterial factor required for autophagy in epithelial-infected cells. Treatment of macrophages with 3-methyladenine, an inhibitor of autophagy, enhanced pyroptosis induced by Shigella infection, suggesting that autophagy protects infected macrophages from pyroptosis. Thus, Ipaf plays a critical role in caspase-1 activation induced by Shigella independently of flagellin. Furthermore, the absence of Ipaf or caspase-1, but not ASC, regulates pyroptosis and the induction of autophagy in Shigella-infected macrophages, providing a novel function for NLR proteins in bacterial-host interactions.
Conflict of interest statement
Figures




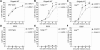
References
-
- Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. - PubMed
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Inohara N, Chamaillard M, McDonald C, Nuñez G. NOD-LRR proteins: Role in host-microbial interactions and inflammatory disease. Annu Rev Biochem. 2005;74:355–383. - PubMed
-
- Ting JP, Davis BK. CATERPILLER: A novel gene family important in immunity, cell death, and diseases. Annu Rev Immunol. 2005;23:387–414. - PubMed
-
- Martinon F, Tschopp J. Inflammatory caspases: Linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561–574. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

