An actin-based wave generator organizes cell motility
- PMID: 17696648
- PMCID: PMC1945041
- DOI: 10.1371/journal.pbio.0050221
An actin-based wave generator organizes cell motility
Abstract
Although many of the regulators of actin assembly are known, we do not understand how these components act together to organize cell shape and movement. To address this question, we analyzed the spatial dynamics of a key actin regulator--the Scar/WAVE complex--which plays an important role in regulating cell shape in both metazoans and plants. We have recently discovered that the Hem-1/Nap1 component of the Scar/WAVE complex localizes to propagating waves that appear to organize the leading edge of a motile immune cell, the human neutrophil. Actin is both an output and input to the Scar/WAVE complex: the complex stimulates actin assembly, and actin polymer is also required to remove the complex from the membrane. These reciprocal interactions appear to generate propagated waves of actin nucleation that exhibit many of the properties of morphogenesis in motile cells, such as the ability of cells to flow around barriers and the intricate spatial organization of protrusion at the leading edge. We propose that cell motility results from the collective behavior of multiple self-organizing waves.
Conflict of interest statement
Figures
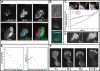
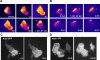

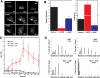


 = 0) freezes Hem-1 waves and increases their intensity, mirroring the behavior of living cells (compare with Figure 4A, Videos S15 and S16). The top panel represents actin polymer, and the bottom panel represents membrane-associated Hem-1.
= 0) freezes Hem-1 waves and increases their intensity, mirroring the behavior of living cells (compare with Figure 4A, Videos S15 and S16). The top panel represents actin polymer, and the bottom panel represents membrane-associated Hem-1.References
-
- Stradal TE, Scita G. Protein complexes regulating Arp2/3-mediated actin assembly. Curr Opin Cell Biol. 2006;18:4–10. - PubMed
-
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. - PubMed
-
- Sun CX, Downey GP, Zhu F, Koh AL, Thang H, et al. Rac1 is the small GTPase responsible for regulating the neutrophil chemotaxis compass. Blood. 2004;104:3758–3765. - PubMed
-
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. - PubMed
-
- Eden S, Rohatgi R, Podtelejnikov AV, Mann M, Kirschner MW. Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature. 2002;418:790–793. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

