Effect of glycation on alpha-crystallin structure and chaperone-like function
- PMID: 17696877
- PMCID: PMC2267351
- DOI: 10.1042/BJ20070989
Effect of glycation on alpha-crystallin structure and chaperone-like function
Abstract
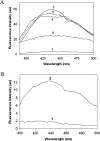
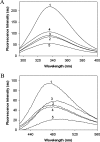
The chaperone-like activity of alpha-crystallin is considered to play an important role in the maintenance of the transparency of the eye lens. However, in the case of aging and in diabetes, the chaperone function of alpha-crystallin is compromized, resulting in cataract formation. Several post-translational modifications, including non-enzymatic glycation, have been shown to affect the chaperone function of alpha-crystallin in aging and in diabetes. A variety of agents have been identified as the predominant sources for the formation of AGEs (advanced glycation end-products) in various tissues, including the lens. Nevertheless, glycation of alpha-crystallin with various sugars has resulted in divergent results. In the present in vitro study, we have investigated the effect of glucose, fructose, G6P (glucose 6-phosphate) and MGO (methylglyoxal), which represent the major classes of glycating agents, on the structure and chaperone function of alpha-crystallin. Modification of alpha-crystallin with all four agents resulted in the formation of glycated protein, increased AGE fluorescence, protein cross-linking and HMM (high-molecular-mass) aggregation. Interestingly, these glycation-related profiles were found to vary with different glycating agents. For instance, CML [N(epsilon)-(carboxymethyl)lysine] was the predominant AGE formed upon glycation of alpha-crystallin with these agents. Although fructose and MGO caused significant conformational changes, there were no significant structural perturbations with glucose and G6P. With the exception of MGO modification, glycation with other sugars resulted in decreased chaperone activity in aggregation assays. However, modification with all four sugars led to the loss of chaperone activity as assessed using an enzyme inactivation assay. Glycation-induced loss of alpha-crystallin chaperone activity was associated with decreased hydrophobicity. Furthermore, alpha-crystallin isolated from glycated TSP (total lens soluble protein) had also increased AGE fluorescence, CML formation and diminished chaperone activity. These results indicate the susceptibility of alpha-crystallin to non-enzymatic glycation by various sugars and their derivatives, whose levels are elevated in diabetes. We also describe the effects of glycation on the structure and chaperone-like activity of alpha-crystallin.
Figures





Similar articles
-
Effect of dicarbonyl-induced browning on alpha-crystallin chaperone-like activity: physiological significance and caveats of in vitro aggregation assays.Biochem J. 2004 Apr 15;379(Pt 2):273-82. doi: 10.1042/BJ20031633. Biochem J. 2004. PMID: 14711370 Free PMC article.
-
Enhanced degradation and decreased stability of eye lens alpha-crystallin upon methylglyoxal modification.Exp Eye Res. 2004 Oct;79(4):577-83. doi: 10.1016/j.exer.2004.07.003. Exp Eye Res. 2004. PMID: 15381041
-
Delay of diabetic cataract in rats by the antiglycating potential of cumin through modulation of alpha-crystallin chaperone activity.J Nutr Biochem. 2009 Jul;20(7):553-62. doi: 10.1016/j.jnutbio.2008.05.015. Epub 2008 Sep 11. J Nutr Biochem. 2009. PMID: 18789666
-
Chaperone-like activity and hydrophobicity of alpha-crystallin.IUBMB Life. 2006 Nov;58(11):632-41. doi: 10.1080/15216540601010096. IUBMB Life. 2006. PMID: 17085382 Review.
-
Modulation of alpha-crystallin chaperone activity: a target to prevent or delay cataract?IUBMB Life. 2009 May;61(5):485-95. doi: 10.1002/iub.176. IUBMB Life. 2009. PMID: 19391162 Review.
Cited by
-
Profiling of lens protease involved in generation of αA-66-80 crystallin peptide using an internally quenched protease substrate.Exp Eye Res. 2013 Apr;109:51-9. doi: 10.1016/j.exer.2013.01.016. Epub 2013 Feb 11. Exp Eye Res. 2013. PMID: 23410823 Free PMC article.
-
Methylglyoxal alters the function and stability of critical components of the protein quality control.PLoS One. 2010 Sep 24;5(9):e13007. doi: 10.1371/journal.pone.0013007. PLoS One. 2010. PMID: 20885985 Free PMC article.
-
Topical Application of Deglycating Enzymes as an Alternative Non-Invasive Treatment for Presbyopia.Int J Mol Sci. 2023 Apr 16;24(8):7343. doi: 10.3390/ijms24087343. Int J Mol Sci. 2023. PMID: 37108506 Free PMC article.
-
Network pharmacology-based study in polymetformin's new function of blocking ages/rage pathway curing diabetic complications.Sci Rep. 2025 Jun 4;15(1):19632. doi: 10.1038/s41598-025-00956-w. Sci Rep. 2025. PMID: 40467581 Free PMC article.
-
Restoring Glutathione Homeostasis in Glycation-Related Eye Diseases: Mechanistic Insights and Therapeutic Interventions Beyond VEGF Inhibition.Antioxidants (Basel). 2025 Jun 14;14(6):731. doi: 10.3390/antiox14060731. Antioxidants (Basel). 2025. PMID: 40563363 Free PMC article. Review.
References
-
- Harding J. J. London: Chapman and Hall; 1991. Cataract: Biochemistry, Epidemiology and Pharmacology.
-
- Derham B. K., Harding J. J. α-Crystallin as a molecular chaperone. Prog. Retin. Eye Res. 1999;18:463–509. - PubMed
-
- Horwitz J. α-Crystallin. Exp. Eye Res. 2003;76:145–153. - PubMed
-
- Reddy G. B., Kumar P. A., Kumar M. S. Chaperone-like activity and hydrophobicity of α-crystallin. IUBMB Life. 2006;58:632–641. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

