Investigation of intratumoural and peritumoural lymphatics expressed by podoplanin and LYVE-1 in the hybridoma-induced tumours
- PMID: 17696907
- PMCID: PMC2517309
- DOI: 10.1111/j.1365-2613.2007.00532.x
Investigation of intratumoural and peritumoural lymphatics expressed by podoplanin and LYVE-1 in the hybridoma-induced tumours
Abstract
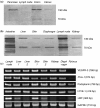
Tumour-associated lymphatics contribute to a key component of metastatic spread, however, the biological interaction of tumour cells with intratumoural and peritumoural lymphatics (ITLs and PTLs) has remained unclear. To address this important issue, we have focused on the morphological and molecular aspects of newly formed lymphatics (lymphangiogenesis) and pre-existing lymphatics in the intratumoural and peritumoural tissues by using a hybridoma-induced tumour model. In the present study, ITLs with very high vessel density within the tumour mass showed small and flattened contours that varied from non-solid-to-solid tumours, whereas PTLs were relatively disorganized and tortuous, and packed with a cluster of tumour cells at the tumour periphery. Lymphatic endothelial cells (LECs) both in ITLs and PTLs were expressed with LYVE-1 and podoplanin in various tumour tissues, in which initial lymphatics were extremely extended and dilated. The tumour cells were frequently detected adhering to or penetrating lymphatic walls, especially near the open junctions. In the metastatic tissues, lymphangiogenic vasculatures occurred within the tumour matrix, and collecting PTLs represented abnormal twisty valve leaflets. The Western blot and RT-PCR analysis showed local variations of LEC proliferating potentials and lymphatic involvement in metastasis by a distinct profile of the protein and mRNA expression by LYVE-1, podoplanin, Prox-1 and vascular endothelial growth factor-3 (VEGFR-3). These findings indicated that both ITLs and PTLs, including enlarged pre-existing and newly formed lymphatics, may play a crucial role in metastasis with an active tumour cell adhesion, invasion, migration and implantation.
Figures
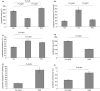

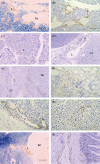


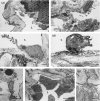
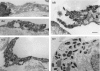
References
-
- Achen MG, McColl BK, Stacker SA. Focus on lymphangiogenesis in tumor metastasis. Cancer Cell. 2005;7:121–127. - PubMed
-
- Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell. 2002;1:219–227. - PubMed
-
- Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. - PubMed
-
- Beasley NJ, Prevo R, Banerji S, et al. Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res. 2002;62:1315–1320. - PubMed
-
- Bjorndahl MA, Cao R, Burton JB, et al. Vascular endothelial growth factor-a promotes peritumoral lymphangiogenesis and lymphatic metastasis. Cancer Res. 2005;65:9261–9268. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

