Chondroprotective effects of a proanthocyanidin rich Amazonian genonutrient reflects direct inhibition of matrix metalloproteinases and upregulation of IGF-1 production by human chondrocytes
- PMID: 17697350
- PMCID: PMC1971260
- DOI: 10.1186/1476-9255-4-16
Chondroprotective effects of a proanthocyanidin rich Amazonian genonutrient reflects direct inhibition of matrix metalloproteinases and upregulation of IGF-1 production by human chondrocytes
Abstract
Background: The Amazonian medicinal plant Sangre de grado (Croton palanostigma) has traditional applications for the treatment of wound healing and inflammation. We sought to characterize two extracts (progrado and zangrado) in terms of safety and oligomeric proanthocyanidin chain length. Additionally progrado was evaluated for antioxidant activity and possible chondroprotective actions.
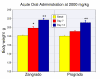
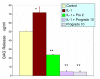
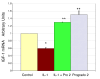
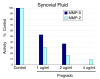
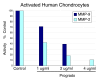
Methods: Acute oral safety and toxicity was tested in rats according under OECD protocol number 420. The profile of proanthocyanidin oligomers was determined by HPLC and progrado's antioxidant activity quantified by the ORAC, NORAC and HORAC assays. Human cartilage explants, obtained from surgical specimens, were used to assess chondroproteciton with activity related to direct inhibitory effects on human matrix metalloproteinase (MMP, gelatinolytic) activity using synovial fluid and chondrocytes activated with IL-1beta (10 ng/ml). Additionally, progrado (2-10 mug/ml) was tested for its ability to maintain optimal IGF-1 transcription and translation in cartilage explants and cultured chondrocytes.
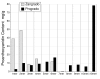
Results: Both progrado and zangrado at doses up to 2000 mg/kg (po) displayed no evidence of toxicity. Oligomeric proanthocyanidin content was high for both progrado (158 mg/kg) and zangrado (124 mg/kg), with zangrado almost entirely composed of short oligomers (<6 mer), whereas the majority of oligomers in progrado exceeded 10 mers. Progrado was a remarkably potent antioxidant in the standardized tests ORAC, NORAC and HORAC. Progrado was exceptionally effective in reducing both basal and IL-1beta induced glycosaminoglycan release from human cartilage explants at concentrations that also directly blocked the gelatinolytic activity of MMP-2 and MMP-9. Progrado prevented IL-1beta induced suppression of IGF-1 production from human cartilage explants as well as stimulating basal IGF-1 production (P < 0.05). Comparable changes in IGF-1 gene expression were noted in cultured human chondrocytes.
Conclusion: Progrado has a promising safety profile, significant chondroprotective and antioxidant actions, directly inhibits MMP activity and promotes the production of the cartilage repair factor, IGF-1. This suggests that progrado may offer therapeutic benefits in joint health, wound healing and inflammation.
Figures

References
-
- Duke J, Vasquez R. Amazonian Ethnobotanical Dictionary. CRC Press, Boca Raton, FL; 1994.
-
- Miller MJS, MacNaughton WK, Zhang X-J, Thompson JH, Charbonnet RM, Bobrowski P, Lao J, Trentacosti AM, Sandoval M. Treatment of gastric ulcers and diarrhea with the amazonian medicinal, sangre de grado. Amer J Physiol. 2000;279:G192–G200. - PubMed
-
- Miller MJS, Vergnolle N, McKnight W, Musah RA, Davison CA, Trentacosti AM, Thompson JH, Sandoval M, Wallace JL. Inhibition of neurogenic inflammation by the amazonian herbal medicine, sangre de grado. J Investigative Dermatology. 2001;117:725–730. doi: 10.1046/j.0022-202x.2001.01446.x. - DOI - PubMed
-
- Miller MJS, Reuter BK, Wallace J, Sharkey K, Bobrowski P. Mechanistic and clinical assessment of zangrado®, an extract of the amazonian ethnomedicine sangre de grado, for the treatment of itch. In: Yosipovitch G, editor. Itch: Basic Mechanisms and Therapy. Marcel Dekker; 2003. pp. 311–320.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

