Validation of rice genome sequence by optical mapping
- PMID: 17697381
- PMCID: PMC2048515
- DOI: 10.1186/1471-2164-8-278
Validation of rice genome sequence by optical mapping
Abstract
Background: Rice feeds much of the world, and possesses the simplest genome analyzed to date within the grass family, making it an economically relevant model system for other cereal crops. Although the rice genome is sequenced, validation and gap closing efforts require purely independent means for accurate finishing of sequence build data.
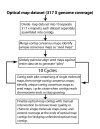
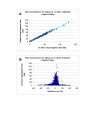
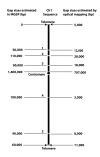
Results: To facilitate ongoing sequencing finishing and validation efforts, we have constructed a whole-genome SwaI optical restriction map of the rice genome. The physical map consists of 14 contigs, covering 12 chromosomes, with a total genome size of 382.17 Mb; this value is about 11% smaller than original estimates. 9 of the 14 optical map contigs are without gaps, covering chromosomes 1, 2, 3, 4, 5, 7, 8 10, and 12 in their entirety - including centromeres and telomeres. Alignments between optical and in silico restriction maps constructed from IRGSP (International Rice Genome Sequencing Project) and TIGR (The Institute for Genomic Research) genome sequence sources are comprehensive and informative, evidenced by map coverage across virtually all published gaps, discovery of new ones, and characterization of sequence misassemblies; all totalling ~14 Mb. Furthermore, since optical maps are ordered restriction maps, identified discordances are pinpointed on a reliable physical scaffold providing an independent resource for closure of gaps and rectification of misassemblies.
Conclusion: Analysis of sequence and optical mapping data effectively validates genome sequence assemblies constructed from large, repeat-rich genomes. Given this conclusion we envision new applications of such single molecule analysis that will merge advantages offered by high-resolution optical maps with inexpensive, but short sequence reads generated by emerging sequencing platforms. Lastly, map construction techniques presented here points the way to new types of comparative genome analysis that would focus on discernment of structural differences revealed by optical maps constructed from a broad range of rice subspecies and varieties.
Figures



References
-
- Soderlund C, Longden I, Mott R. FPC: a system for building contigs from restriction fingerprinted clones. Comput Appl Biosci. 1997;13:523–535. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

