Mechanisms of palatal epithelial seam disintegration by transforming growth factor (TGF) beta3
- PMID: 17698055
- PMCID: PMC2084085
- DOI: 10.1016/j.ydbio.2007.06.018
Mechanisms of palatal epithelial seam disintegration by transforming growth factor (TGF) beta3
Abstract
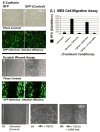
TGFbeta3 signaling initiates and completes sequential phases of cellular differentiation that is required for complete disintegration of the palatal medial edge seam, that progresses between 14 and 17 embryonic days in the murine system, which is necessary in establishing confluence of the palatal stroma. Understanding the cellular mechanism of palatal MES disintegration in response to TGFbeta3 signaling will result in new approaches to defining the causes of cleft palate and other facial clefts that may result from failure of seam disintegration. We have isolated MES primary cells to study the details of MES disintegration mechanism by TGFbeta3 during palate development using several biochemical and genetic approaches. Our results demonstrate a novel mechanism of MES disintegration where MES, independently yet sequentially, undergoes cell cycle arrest, cell migration and apoptosis to generate immaculate palatal confluency during palatogenesis in response to robust TGFbeta3 signaling. The results contribute to a missing fundamental element to our base knowledge of the diverse roles of TGFbeta3 in functional and morphological changes that MES undergo during palatal seam disintegration. We believe that our findings will lead to more effective treatment of facial clefting.
Figures










References
-
- Bahi N, Zhang J, Llovera M, Ballester M, Comella JX, Sanchis D. Switch from caspase-dependent to caspase-independent death during heart development: essential role of endonuclease G in ischemia-induced DNA processing of differentiated cardiomyocytes. J Biol Chem. 2006;281:22943–52. - PubMed
-
- Boehm M, Nabel EG. Cell cycle and cell migration: new pieces to the puzzle. Circulation. 2001;103:2879–81. - PubMed
-
- Bonneton C, Sibarita JB, Thiery JP. Relationship between cell migration and cell cycle during the initiation of epithelial to fibroblastoid transition. Cell Motil Cytoskeleton. 1999;43:288–95. - PubMed
-
- Boyer AS, Ayerinskas II, Vincent EB, McKinney LA, Weeks DL, Runyan RB. TGFbeta2 and TGFbeta3 have separate and sequential activities during epithelial-mesenchymal cell transformation in the embryonic heart. Dev Biol. 1999;208:530–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

